Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-11-04 , DOI: 10.1016/j.cej.2020.127604 Wenda Shi , Jianbo Guo , Caicai Lu , Zhi Chen , Haibo Li , Yuanyuan Song , Yi Han , Yanan Hou
|
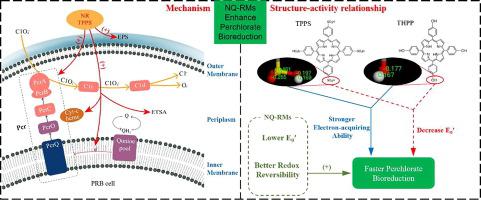
The effect of various non-quinone redox mediators (NQ-RMs) on the enhancement of perchlorate bioreduction were investigated in this study, with analysis of the structure-activity relationship and mechanism. In the presence of Netural Red (NR), Riboflavin, Meso-tetrakis (4-sulfonatophenyl) porphyrin (TPPS), Meso-Tetraphenylporphyrin (TPP) and 5,10,15,20-tetrakis (4-hydroxyphenyl) porphyrin (THPP), perchlorate bioreduction rates were enhanced 1.40, 1.23, 1.67, 1.57 and 1.64-fold at 48 h, respectively. Structure-activity relationship analysis showed that NQ-RMs with increasingly negative redox potentials and greater redox reversibility were more conducive to perchlorate bioreduction. According to inductive effect, conjugated effect and Mulliken population analysis, substituents can affect the perchlorate bioreduction efficiency of NQ-RMs with similar chemical structures. Cyclic voltammetry and Tafel plot analysis demonstrated that the electron transfer rate was accelerated by NR and TPPS. The increased extracellular polymeric substance (EPS) secretion and electron transfer system activity proved that cell metabolic activity was promoted by NR and TPPS. Moreover, perchlorate reductase activity was enhanced by NR and TPPS. As a result, the possible mechanism for enhancement of perchlorate bioreduction by NR and TPPS is the acceleration of electron transfer, promoting metabolic activity and enhancing perchlorate reductase activity. This study proposed a strategy for screening of NQ-RMs and provided reference for improving the efficiency of perchlorate pollutants bioreduction.
中文翻译:

非醌氧化还原介质增强高氯酸盐生物还原:作用,构效关系和机制。
通过分析其结构活性关系和机理,研究了各种非醌氧化还原介体(NQ-RMs)对高氯酸盐生物还原作用的影响。在存在网红(NR),核黄素,中四(4-磺酰基苯基)卟啉(TPPS),中四苯基卟啉(TPP)和5,10,15,20-四(4-羟基苯基)卟啉(THPP)的情况下在48小时时,高氯酸盐的生物还原率分别提高了1.40、1.23、1.67、1.57和1.64倍。结构-活性关系分析表明,氧化还原电位越来越负且氧化还原可逆性更高的NQ-RM更有利于高氯酸盐的生物还原。根据归纳效应,共轭效应和Mulliken群体分析,取代基会影响具有相似化学结构的NQ-RM的高氯酸盐生物还原效率。循环伏安法和Tafel图分析表明,NR和TPPS可加速电子转移速率。增加的细胞外聚合物(EPS)分泌和电子转移系统活性证明了NR和TPPS促进了细胞代谢活性。此外,NR和TPPS增强了高氯酸盐还原酶的活性。结果,通过NR和TPPS增强高氯酸盐生物还原的可能机制是加速电子转移,促进代谢活性和增强高氯酸盐还原酶活性。这项研究提出了一种筛选NQ-RMs的策略,并为提高高氯酸盐污染物生物还原效率提供了参考。循环伏安法和Tafel图分析表明,NR和TPPS可加速电子转移速率。增加的细胞外聚合物(EPS)分泌和电子转移系统活性证明了NR和TPPS促进了细胞代谢活性。此外,NR和TPPS增强了高氯酸盐还原酶的活性。结果,通过NR和TPPS增强高氯酸盐生物还原的可能机制是加速电子转移,促进代谢活性和增强高氯酸盐还原酶活性。这项研究提出了一种筛选NQ-RMs的策略,并为提高高氯酸盐污染物生物还原效率提供了参考。循环伏安法和Tafel图分析表明,NR和TPPS可加速电子转移速率。增加的细胞外聚合物(EPS)分泌和电子转移系统活性证明了NR和TPPS促进了细胞代谢活性。此外,NR和TPPS增强了高氯酸盐还原酶的活性。结果,通过NR和TPPS增强高氯酸盐生物还原的可能机制是加速电子转移,促进代谢活性和增强高氯酸盐还原酶活性。这项研究提出了一种筛选NQ-RMs的策略,并为提高高氯酸盐污染物生物还原效率提供了参考。增加的细胞外聚合物(EPS)分泌和电子转移系统活性证明了NR和TPPS促进了细胞代谢活性。此外,NR和TPPS增强了高氯酸盐还原酶的活性。结果,通过NR和TPPS增强高氯酸盐生物还原的可能机制是加速电子转移,促进代谢活性和增强高氯酸盐还原酶活性。这项研究提出了一种筛选NQ-RMs的策略,并为提高高氯酸盐污染物生物还原效率提供了参考。增加的细胞外聚合物(EPS)分泌和电子转移系统活性证明了NR和TPPS促进了细胞代谢活性。此外,NR和TPPS增强了高氯酸盐还原酶的活性。结果,通过NR和TPPS增强高氯酸盐生物还原的可能机制是加速电子转移,促进代谢活性和增强高氯酸盐还原酶活性。这项研究提出了一种筛选NQ-RMs的策略,并为提高高氯酸盐污染物生物还原效率提供了参考。NR和TPPS增强高氯酸盐生物还原的可能机制是加速电子转移,促进代谢活性和增强高氯酸盐还原酶活性。这项研究提出了一种筛选NQ-RMs的策略,并为提高高氯酸盐污染物生物还原效率提供了参考。NR和TPPS增强高氯酸盐生物还原的可能机制是加速电子转移,促进代谢活性和增强高氯酸盐还原酶活性。这项研究提出了一种筛选NQ-RMs的策略,并为提高高氯酸盐污染物生物还原效率提供了参考。











































 京公网安备 11010802027423号
京公网安备 11010802027423号