Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-11-04 , DOI: 10.1016/j.bmc.2020.115835 Michael J V da Silva 1 , Andrey P Jacomini 1 , Mariana C Figueiredo 2 , Davi F Back 3 , Mary A Foglio 2 , Ana L T G Ruiz 4 , Fávero R Paula 5 , Fernanda A Rosa 1
|
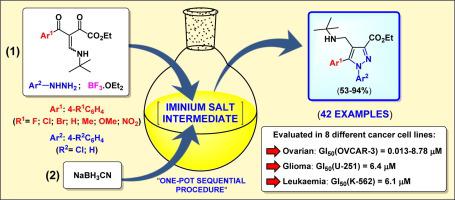
A new one-pot two-step sequential methodology for synthesis of novel 3-carboxyethyl 4-[(tert-butylamino)methyl]-N-arylpyrazole derivatives is reported. One-pot transformation of β-enamino diketones and arylhydrazines generated 4-iminium-N-arylpyrazole salt intermediates in situ, which were easily transformed into 4-[(tert-butylamino)methyl]-N-arylpyrazole derivatives by NaBH3CN. The products could be isolated in the free or hydrochloride salt forms. Also, it was possible to obtain the products in the zwitterionic form by ester group hydrolysis. Furthermore, all synthesised compounds were evaluated in vitro against a panel of eight human tumor cell lines. The 4-[(tert-butylamino)methyl]-N-arylpyrazole derivatives were much more powerful than the hydrochloride and zwitterionic forms. Moreover, the results suggest that the N-aryl group at the pyrazole ring is vital for modulating antiproliferative activity. The 3-carboxyethyl 4-[(tert-butylamino)methyl]-N-phenylpyrazoles 3a–g exhibited higher inhibitory activities against OVCAR-3, with GI50 values of 0.013–8.78 μM, and lower inhibitory activities against normal human cell lines. Molecular docking was performed to evaluate the probable binding mode of 3a into active site of CDK2.
中文翻译:

4-氨基甲基-N-芳基吡唑的高效合成和抗肿瘤评价:发现有效的卵巢癌选择性药物
报道了一种用于合成新型 3-羧乙基 4-[(叔丁基氨基)甲基] -N-芳基吡唑衍生物的新的一锅两步顺序方法。β-烯氨基二酮和芳基肼的一锅转化原位生成 4-亚胺-N-芳基吡唑盐中间体,其很容易通过 NaBH 3转化为 4-[(叔丁基氨基)甲基] -N-芳基吡唑衍生物CN. 产物可以游离盐或盐酸盐形式分离。此外,可以通过酯基水解获得两性离子形式的产物。此外,所有合成的化合物都针对一组八种人类肿瘤细胞系进行了体外评估。4-[(叔丁基氨基)甲基] -N-芳基吡唑衍生物比盐酸盐和两性离子形式强得多。此外,结果表明吡唑环上的N-芳基对于调节抗增殖活性至关重要。3-羧乙基4-[(叔丁基氨基)甲基] -N-苯基吡唑3a - g对 OVCAR-3 表现出较高的抑制活性,GI 50值为 0.013–8.78 μM,对正常人细胞系的抑制活性较低。进行分子对接以评估3a与 CDK2 活性位点的可能结合模式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号