Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2020-11-04 , DOI: 10.1016/j.apcata.2020.117909 Miquel García-Bofill , Peter W. Sutton , Marina Guillén , Gregorio Álvaro
|
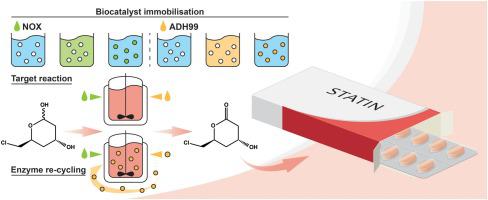
Statins inhibit the synthesis of LDL-cholesterol which is related to cardiovascular diseases. One of the key steps in the synthesis of the chiral side chain of some statins is the oxidation of the chlorolactol to chlorolactone. This oxidation has been performed by an alcohol dehydrogenase (ADH99) using a NADPH-oxidase (NOX) as a cofactor regeneration system. The reaction conditions were optimised obtaining high reaction yield (94.7 %), space time yield (4.6 g L−1 h−1) and biocatalyst yield (7.9 mg product mg−1 biocatalyst). Both enzymes have been efficiently immobilised onto different supports (Eupergit® CM, Amino-agarose, Epoxy agarose-UAB, Purolite ECR8409 and ECR8415). ADH99 showed a stability improvement when immobilised. However, NOX did not show any significant stability enhancement. The most stable ADH99 immobilised derivative, ADH99-Epoxy agarose-UAB, was used to perform the oxidation, improving 1.5-fold both, the total amount of product produced and the biocatalyst yield compared to the ADH99 soluble form.
中文翻译:

以NADPH氧化酶为辅因子再生体系的固定化醇脱氢酶酶促合成他汀类药物前体
他汀类药物抑制与心血管疾病有关的LDL-胆固醇的合成。某些他汀类药物的手性侧链合成中的关键步骤之一是将氯丙醇氧化为氯内酯。该氧化已经通过使用NADPH-氧化酶(NOX)作为辅因子再生系统的醇脱氢酶(ADH99)进行。优化了反应条件,获得了高反应收率(94.7%),时空收率(4.6 g L -1 h -1)和生物催化剂收率(7.9 mg产品mg -1生物催化剂)。两种酶已被有效地固定在不同的载体上(CM,氨基琼脂糖,环氧琼脂糖-UAB,Purilite ECR8409和ECR8415)。固定后,ADH99表现出稳定性的提高。但是,NOX没有显示出任何明显的稳定性增强。与ADH99可溶性形式相比,最稳定的ADH99固定化衍生物ADH99-环氧琼脂糖-UAB进行了氧化,将产物的总量和生物催化剂产率提高了1.5倍。











































 京公网安备 11010802027423号
京公网安备 11010802027423号