当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemical Synthesis of [2H]-Ethyl Tosylate and Exploration of Its Crypto-optically Active Character Combining Complementary Spectroscopic Tools
Organic Letters ( IF 5.2 ) Pub Date : 2020-11-03 , DOI: 10.1021/acs.orglett.0c03219 Timothée Naret 1 , Philippe Lesot 2 , Andrew R. Puente 3 , Prasad L. Polavarapu 3 , David-Alexandre Buisson 1 , Jeanne Crassous 4 , Grégory Pieters 1 , Sophie Feuillastre 1
Organic Letters ( IF 5.2 ) Pub Date : 2020-11-03 , DOI: 10.1021/acs.orglett.0c03219 Timothée Naret 1 , Philippe Lesot 2 , Andrew R. Puente 3 , Prasad L. Polavarapu 3 , David-Alexandre Buisson 1 , Jeanne Crassous 4 , Grégory Pieters 1 , Sophie Feuillastre 1
Affiliation
|
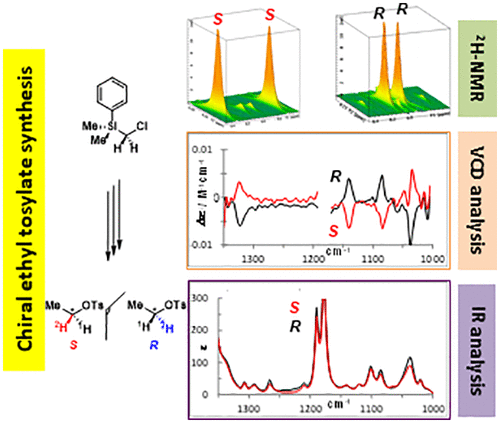
Small chiral molecules are excellent candidates to push the boundaries of enantiodiscrimination analytical techniques. Here is reported the synthesis of two new deuterated chiral probes, (R)- and (S)-[2H]-ethyl tosylate, obtained with high enantiomeric excesses. Due to their crypto-optically active properties, the discrimination of each enantiomer is challenging. Whereas their enantiopurity is determined by 2H NMR in chiral anisotropic media, their identification was performed by combining quantum chemical calculations and vibrational circular dichroism analysis.
中文翻译:

[ 2 H]-甲苯磺酸乙酯的化学合成及其结合互补光谱工具的隐密码活性特征的探索
小手性分子是突破对映歧视技术的极好选择。在此报道了以高对映体过量获得的两种新的氘代手性探针,(R)-和(S)-[ 2 H]-甲苯磺酸乙酯的合成。由于它们具有密码光学活性,因此区分每种对映异构体具有挑战性。尽管它们的对映体纯度是通过2 H NMR在手性各向异性介质中确定的,但其鉴定是通过结合量子化学计算和振动圆二色性分析来进行的。
更新日期:2020-11-21
中文翻译:

[ 2 H]-甲苯磺酸乙酯的化学合成及其结合互补光谱工具的隐密码活性特征的探索
小手性分子是突破对映歧视技术的极好选择。在此报道了以高对映体过量获得的两种新的氘代手性探针,(R)-和(S)-[ 2 H]-甲苯磺酸乙酯的合成。由于它们具有密码光学活性,因此区分每种对映异构体具有挑战性。尽管它们的对映体纯度是通过2 H NMR在手性各向异性介质中确定的,但其鉴定是通过结合量子化学计算和振动圆二色性分析来进行的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号