当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unraveling the NaCl Concentration Effect on the First Stages of α-Synuclein Aggregation
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-11-03 , DOI: 10.1021/acs.biomac.0c01292 Rafael Ramis 1, 2 , Joaquín Ortega-Castro 1, 2 , Bartolomé Vilanova 1, 2 , Miquel Adrover 1, 2 , Juan Frau 1, 2
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-11-03 , DOI: 10.1021/acs.biomac.0c01292 Rafael Ramis 1, 2 , Joaquín Ortega-Castro 1, 2 , Bartolomé Vilanova 1, 2 , Miquel Adrover 1, 2 , Juan Frau 1, 2
Affiliation
|
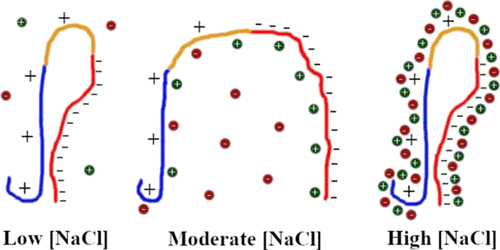
Intraneuronal aggregation of the intrinsically disordered protein α-synuclein is at the core of Parkinson’s disease and related neurodegenerative disorders. Several reports show that the concentration of salts in the medium heavily affects its aggregation rate and fibril morphology, but a characterization of the individual monomeric conformations underlying these effects is still lacking. In this work, we have applied our α-synuclein-optimized coarse-grained molecular dynamics approach to decipher the structural features of the protein monomer under a range of NaCl concentrations (0.0−1.0 M). The results show that key intramolecular contacts between the terminal domains are lost at intermediate concentrations (leading to extended conformations likely to fibrillate), but recovered at high concentrations (leading to compact conformations likely to evolve toward amorphous aggregates). The pattern of direct interactions of the terminal α-synuclein domains with Na+ and Cl– ions plays a key role in explaining this effect. Our results are consistent with a recent study reporting a fibrillation enhancement at moderate NaCl concentrations but an inhibition at higher concentrations. The present work will contribute to improving our understanding of the structural features of monomeric α-synuclein, determining its NaCl-induced fibrillation propensity and the molecular basis of synucleinopathies, necessary for the future development of disease-halting therapies.
中文翻译:

揭示NaCl浓度对α-突触核蛋白聚集初期的影响
本质上失调的蛋白质α-突触核蛋白的神经内神经聚集是帕金森氏病和相关神经退行性疾病的核心。几份报告表明,培养基中盐的浓度会严重影响其聚集速率和原纤维形态,但仍缺乏表征这些作用的单个单体构象的特征。在这项工作中,我们应用了我们的α-突触核蛋白优化的粗粒分子动力学方法来解密在一定浓度的NaCl(0.0-1.0 M)下蛋白质单体的结构特征。结果表明,末端域之间的关键分子内接触在中等浓度时会丢失(导致可能发生原纤化的扩展构象),但可以高浓度回收(导致紧密构象可能演变为无定形聚集体)。末端α-突触核蛋白域与Na直接相互作用的模式+和Cl -离子在解释这种效果的关键作用。我们的结果与最近的一项研究一致,该研究报道了在适度的NaCl浓度下纤维化可增强,但在更高的浓度下具有抑制作用。当前的工作将有助于增进我们对单体α-突触核蛋白的结构特征的了解,确定其NaCl诱导的原纤维形成倾向和突触核蛋白病的分子基础,这对于疾病终止疗法的未来发展是必需的。
更新日期:2020-12-14
中文翻译:

揭示NaCl浓度对α-突触核蛋白聚集初期的影响
本质上失调的蛋白质α-突触核蛋白的神经内神经聚集是帕金森氏病和相关神经退行性疾病的核心。几份报告表明,培养基中盐的浓度会严重影响其聚集速率和原纤维形态,但仍缺乏表征这些作用的单个单体构象的特征。在这项工作中,我们应用了我们的α-突触核蛋白优化的粗粒分子动力学方法来解密在一定浓度的NaCl(0.0-1.0 M)下蛋白质单体的结构特征。结果表明,末端域之间的关键分子内接触在中等浓度时会丢失(导致可能发生原纤化的扩展构象),但可以高浓度回收(导致紧密构象可能演变为无定形聚集体)。末端α-突触核蛋白域与Na直接相互作用的模式+和Cl -离子在解释这种效果的关键作用。我们的结果与最近的一项研究一致,该研究报道了在适度的NaCl浓度下纤维化可增强,但在更高的浓度下具有抑制作用。当前的工作将有助于增进我们对单体α-突触核蛋白的结构特征的了解,确定其NaCl诱导的原纤维形成倾向和突触核蛋白病的分子基础,这对于疾病终止疗法的未来发展是必需的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号