当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Quantifying the Selectivity of Protein–Protein and Small Molecule Interactions with Fluorinated Tandem Bromodomain Reader Proteins
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-11-03 , DOI: 10.1021/acschembio.0c00720 Prakriti Kalra 1 , Logan McGraw 1 , Jennifer R Kimbrough 1 , Anil K Pandey 1 , Jonathan Solberg 2 , Huarui Cui 1 , Anand Divakaran 3 , Kristen John 2 , Jon E Hawkinson 2, 3 , William C K Pomerantz 1, 3
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-11-03 , DOI: 10.1021/acschembio.0c00720 Prakriti Kalra 1 , Logan McGraw 1 , Jennifer R Kimbrough 1 , Anil K Pandey 1 , Jonathan Solberg 2 , Huarui Cui 1 , Anand Divakaran 3 , Kristen John 2 , Jon E Hawkinson 2, 3 , William C K Pomerantz 1, 3
Affiliation
|
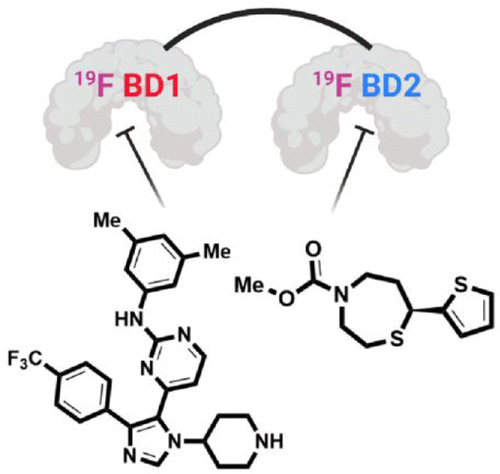
Multidomain bromodomain-containing proteins regulate gene expression via chromatin binding, interactions with the transcriptional machinery, and by recruiting enzymatic activity. Selective inhibition of members of the bromodomain and extra-terminal (BET) family is important to understand their role in disease and gene regulation, although due to the similar binding sites of BET bromodomains, selective inhibitor discovery has been challenging. To support the bromodomain inhibitor discovery process, here we report the first application of protein-observed fluorine (PrOF) NMR to the tandem bromodomains of BRD4 and BRDT to quantify the selectivity of their interactions with acetylated histones as well as small molecules. We further determine the selectivity profile of a new class of ligands, 1,4-acylthiazepanes, and find them to have ≥3–10-fold selectivity for the C-terminal bromodomain of both BRD4 and BRDT. Given the speed and lower protein concentration required over traditional protein-observed NMR methods, we envision that these fluorinated tandem proteins may find use in fragment screening and evaluating nucleosome and transcription factor interactions.
中文翻译:

用氟化串联溴结构域阅读器蛋白量化蛋白质-蛋白质和小分子相互作用的选择性
含多结构域溴结构域的蛋白质通过染色质结合、与转录机制的相互作用以及募集酶活性来调节基因表达。溴结构域和末端外 (BET) 家族成员的选择性抑制对于了解它们在疾病和基因调控中的作用很重要,尽管由于 BET 溴结构域的结合位点相似,选择性抑制剂的发现一直具有挑战性。为了支持溴结构域抑制剂的发现过程,我们在此报告了首次将蛋白质观察到的氟 (PrOF) NMR 应用于 BRD4 和 BRDT 的串联溴结构域,以量化它们与乙酰化组蛋白以及小分子相互作用的选择性。我们进一步确定了一类新的配体 1,4-酰基硫氮杂的选择性特征,并发现它们对 BRD4 和 BRDT 的 C 末端溴结构域具有≥3-10 倍的选择性。鉴于传统蛋白质观察 NMR 方法所需的速度和较低的蛋白质浓度,我们设想这些氟化串联蛋白质可用于片段筛选和评估核小体和转录因子相互作用。
更新日期:2020-11-21
中文翻译:

用氟化串联溴结构域阅读器蛋白量化蛋白质-蛋白质和小分子相互作用的选择性
含多结构域溴结构域的蛋白质通过染色质结合、与转录机制的相互作用以及募集酶活性来调节基因表达。溴结构域和末端外 (BET) 家族成员的选择性抑制对于了解它们在疾病和基因调控中的作用很重要,尽管由于 BET 溴结构域的结合位点相似,选择性抑制剂的发现一直具有挑战性。为了支持溴结构域抑制剂的发现过程,我们在此报告了首次将蛋白质观察到的氟 (PrOF) NMR 应用于 BRD4 和 BRDT 的串联溴结构域,以量化它们与乙酰化组蛋白以及小分子相互作用的选择性。我们进一步确定了一类新的配体 1,4-酰基硫氮杂的选择性特征,并发现它们对 BRD4 和 BRDT 的 C 末端溴结构域具有≥3-10 倍的选择性。鉴于传统蛋白质观察 NMR 方法所需的速度和较低的蛋白质浓度,我们设想这些氟化串联蛋白质可用于片段筛选和评估核小体和转录因子相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号