当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stabilization of Catalytic Surfaces through Core–Shell Structures: Ag–Ir/Al2O3 Case Study
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-11-03 , DOI: 10.1021/acscatal.0c03297 M. Parizad 1 , A. P. Wong 1 , A. C. Reber 2 , J. M. M. Tengco 1 , S. G. Karakalos 1 , S. N. Khanna 2 , J. R. Regalbuto 1 , J. R. Monnier 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-11-03 , DOI: 10.1021/acscatal.0c03297 M. Parizad 1 , A. P. Wong 1 , A. C. Reber 2 , J. M. M. Tengco 1 , S. G. Karakalos 1 , S. N. Khanna 2 , J. R. Regalbuto 1 , J. R. Monnier 1
Affiliation
|
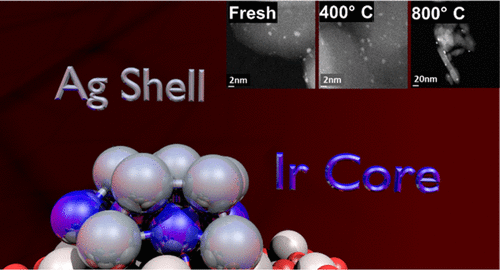
To solve the challenges of catalyst stability and activity at high temperatures, catalyst composition and method of preparation are critical. In this context, the well-established methods of strong electrostatic adsorption and electroless deposition (ED) have been used to synthesize highly dispersed Ag/Ir on different alumina supports (δ,θ-Al2O3 and γ-Al2O3). The surface free energy (SFE) of Ag (1302 erg/cm2) is much lower than that of Ir (3231 erg/cm2); surface thermodynamics dictate the Ir–Ag system should arrange to give the lowest free energy of an Ag layer localized on the Ir surface to minimize the high SFE of Ir. The catalysts remained dispersed at high-temperature annealing treatments (400, 600, and 800 °C). For all Ag–Ir/δ,θ-Al2O3 samples, H2 chemisorption values were higher after annealing at 400 and 600 °C than at 200 °C and were considerably higher than for the corresponding 1.0 wt % Ir/δ,θ-Al2O3 used as the base catalyst. X-ray diffraction data and scanning transmission electron microscopy images indicate that both monometallic catalysts sintered, but deposition of an Ag shell by ED prevented the sintering of both Ag and Ir. Temperature-programmed desorption of H2 measurements corroborates the high H2 uptake chemisorption experiments and indicated the additional H2 capacity was because of more weakly bound H. Computational and X-ray photoelectron spectroscopy results suggest the excess H2 chemisorption can be accounted for by binding up to four H atoms to single-surface Ir atoms surrounded by Ag in the shell of the bimetallic catalysts that have been pretreated at temperatures >400 °C. As a result, H2 capacity increases from the normal adsorption stoichiometry of H/Ir = 1:1 up to as high as 4:1.
中文翻译:

通过核-壳结构稳定催化表面的研究:Ag-Ir / Al 2 O 3案例研究
为了解决催化剂在高温下的稳定性和活性的挑战,催化剂的组成和制备方法至关重要。在这种情况下,成熟的强的静电吸附和无电沉积(ED)的方法已被用于合成高分散的Ag / IR上不同氧化铝载体(δ,θ-Al系2 ö 3和γ-Al系2 ö 3) 。Ag(1302 erg / cm 2)的表面自由能(SFE)远远低于Ir(3231 erg / cm 2)的表面自由能); 表面热力学要求Ir-Ag系统应安排使Ir表面上的Ag层的自由能最低,以最大程度地降低Ir的高SFE。在高温退火处理(400、600和800°C)下,催化剂保持分散状态。对于所有的Ag-IR /δ,θ-Al系2个ö 3样品,H 2吸附值退火后,在400和600℃下比在200℃下均较高和比对相应的1.0重量%的Ir /δ相当高, θ-Al系2 ö 3用作基础催化剂。X射线衍射数据和扫描透射电子显微镜图像表明两种单金属催化剂均被烧结,但是通过ED沉积Ag壳层阻止了Ag和Ir的烧结。H 2测量值的程序升温脱附证实了高H 2吸收化学吸附实验的结果,并表明额外的H 2容量是由于结合的H较弱。计算和X射线光电子能谱结果表明,过量的H 2化学吸附可通过以下途径解决:在> 400°C的温度下进行预处理的双金属催化剂壳中,最多将四个H原子与被Ag包围的单表面Ir原子结合。结果,H 2 容量从正常的吸附化学计量比(H / Ir = 1:1)增加到高达4:1。
更新日期:2020-11-21
中文翻译:

通过核-壳结构稳定催化表面的研究:Ag-Ir / Al 2 O 3案例研究
为了解决催化剂在高温下的稳定性和活性的挑战,催化剂的组成和制备方法至关重要。在这种情况下,成熟的强的静电吸附和无电沉积(ED)的方法已被用于合成高分散的Ag / IR上不同氧化铝载体(δ,θ-Al系2 ö 3和γ-Al系2 ö 3) 。Ag(1302 erg / cm 2)的表面自由能(SFE)远远低于Ir(3231 erg / cm 2)的表面自由能); 表面热力学要求Ir-Ag系统应安排使Ir表面上的Ag层的自由能最低,以最大程度地降低Ir的高SFE。在高温退火处理(400、600和800°C)下,催化剂保持分散状态。对于所有的Ag-IR /δ,θ-Al系2个ö 3样品,H 2吸附值退火后,在400和600℃下比在200℃下均较高和比对相应的1.0重量%的Ir /δ相当高, θ-Al系2 ö 3用作基础催化剂。X射线衍射数据和扫描透射电子显微镜图像表明两种单金属催化剂均被烧结,但是通过ED沉积Ag壳层阻止了Ag和Ir的烧结。H 2测量值的程序升温脱附证实了高H 2吸收化学吸附实验的结果,并表明额外的H 2容量是由于结合的H较弱。计算和X射线光电子能谱结果表明,过量的H 2化学吸附可通过以下途径解决:在> 400°C的温度下进行预处理的双金属催化剂壳中,最多将四个H原子与被Ag包围的单表面Ir原子结合。结果,H 2 容量从正常的吸附化学计量比(H / Ir = 1:1)增加到高达4:1。











































 京公网安备 11010802027423号
京公网安备 11010802027423号