Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
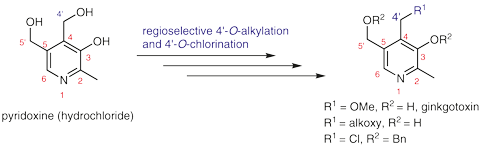
Effective and Versatile Synthesis of Ginkgotoxin and Its 4′-O-Derivatives through Regioselective 4′-O-Alkylation and 4′-O-Chlorination of 3,5′-O-Dibenzylpyridoxine
SynOpen ( IF 2.0 ) Pub Date : 2020-08-14 , DOI: 10.1055/s-0040-1707242 Yan Zhang 1, 2 , Boshi Huang 1 , Richmond Danso-Danquah 1, 2 , Martin K. Safo 1, 2
中文翻译:

通过区域选择性4'-O-烷基化和4'-O-氯化3,5'-O-二苄基吡啶氧有效有效地合成银杏毒素及其4'-O衍生物
更新日期:2020-08-14
SynOpen ( IF 2.0 ) Pub Date : 2020-08-14 , DOI: 10.1055/s-0040-1707242 Yan Zhang 1, 2 , Boshi Huang 1 , Richmond Danso-Danquah 1, 2 , Martin K. Safo 1, 2
Affiliation
|
A regioselective synthesis of ginkgotoxin and its derivatives is described. A blocking–deblocking strategy was employed in this new methodology, which relied on selective ketal protection of the 3- and 4′-hydroxy groups of pyridoxine. The key intermediate, O-dibenzylpyridoxine, was prepared in four steps with a reasonable yield. The present synthetic route enables convenient and versatile preparation of diversified 4′-substituted pyridoxine derivatives.
中文翻译:

通过区域选择性4'-O-烷基化和4'-O-氯化3,5'-O-二苄基吡啶氧有效有效地合成银杏毒素及其4'-O衍生物
描述了银蛋白毒素及其衍生物的区域选择性合成。在这种新方法中采用了封闭-解封闭策略,该策略依赖于吡ido醇的3和4'-羟基的选择性缩酮保护。分四步以合理的收率制备了关键中间体O-二苄基吡ox醇。本合成途径使得能够方便且通用地制备多样化的4'-取代的吡ido醇衍生物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号