Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Surinamensinols A and B
SynOpen ( IF 2.0 ) Pub Date : 2020-10-15 , DOI: 10.1055/s-0040-1707325 Ahmed Al-Harrasi 1 , Satya Kumar Avula 1 , Biswanath Das 1 , Rene Csuk 2 , Ahmed Al-Rawahi 1
中文翻译:

Surinamensinols A和B的全合成
更新日期:2020-11-03
SynOpen ( IF 2.0 ) Pub Date : 2020-10-15 , DOI: 10.1055/s-0040-1707325 Ahmed Al-Harrasi 1 , Satya Kumar Avula 1 , Biswanath Das 1 , Rene Csuk 2 , Ahmed Al-Rawahi 1
Affiliation
|
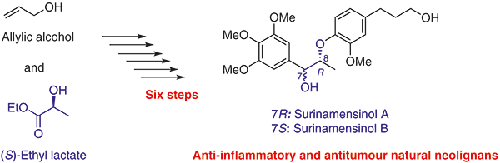
An efficient total synthesis of the naturally occurring anti-inflammatory and antitumour 8-O-4′-neolignans, surinamensinols A and B, has been accomplished from commercially available allyl alcohol and (S)-ethyl lactate. The synthetic sequence involves a palladium-catalysed Suzuki–Miyaura cross-coupling reaction followed by a chiral Mitsunobu reaction as the key steps. This is the first report of the simultaneous stereoselective total synthesis of surinamensinols A and B through a single approach involving only six steps.
中文翻译:

Surinamensinols A和B的全合成
天然存在的抗炎和抗肿瘤的8 - O -4'-新木质素,苏那烯棉酚A和B的有效的全合成已经由市售的烯丙醇和(S)-乳酸乙酯完成。合成过程涉及钯催化的Suzuki-Miyaura交叉偶联反应,然后进行关键的手性Mitsunobu反应。这是关于surinamensinols A和B同时进行立体选择性全合成的首次报道,该方法仅涉及六个步骤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号