Synthesis ( IF 2.2 ) Pub Date : 2020-11-02 , DOI: 10.1055/s-0040-1706547 Shoko Yamazaki 1 , Zhichao Wang 2 , Kentaro Iwata 1 , Khotaro Katayama 1 , Hirotaka Sugiura 2 , Yuji Mikata 3 , Tsumoru Morimoto 4 , Akiya Ogawa 2
|
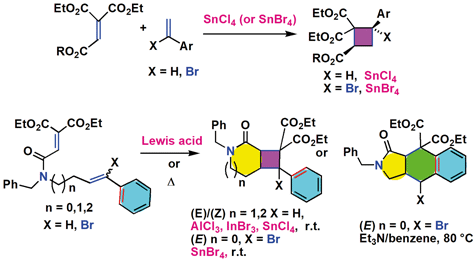
Inter- and intramolecular cycloaddition reactions of ethenetricarboxylates with styrenes and α-halostyrenes have been investigated. The reactions of ethenetricarboxylates with styrenes or α-bromostyrenes in the presence of SnCl4 or SnBr4 stereoselectively gave 2,4-cis-substituted cyclobutanes. The intramolecular cycloaddition reactions of a series of styrene-functionalized ethenetricarboxylate amides, including in situ generated derivatives, showed high diversity of reaction modes depending on the structures and substituents of the substrates. The regioselectivity and stereoselectivity of the reactions as well as reaction mechanisms were discussed based on the DFT calculations.
中文翻译:

乙烯羧酸与苯乙烯和卤代苯乙烯的分子间和分子内环加成反应
已经研究了三羧酸乙烯酯与苯乙烯和α-卤代苯乙烯的分子间和分子内环加成反应。在SnCl 4或SnBr 4的存在下,三羧酸乙烯酯与苯乙烯或α-溴苯乙烯的反应有选择地产生2,4-顺式取代的环丁烷。一系列苯乙烯官能化的乙烯三羧酸酰胺(包括原位生成的衍生物)的分子内环加成反应根据底物的结构和取代基显示出高的反应模式多样性。基于DFT计算,讨论了反应的区域选择性和立体选择性以及反应机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号