Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
DBU-Promoted Formal [4+2] Annulation Reactions of o-Chloromethyl Anilines with Azlactones
Synthesis ( IF 2.2 ) Pub Date : 2020-11-02 , DOI: 10.1055/s-0040-1706549 Jianfeng Xu 1 , Haojie Ji 1 , Chonglong He 1 , Hongjie Gao 2 , Weijun Fu 3
中文翻译:

DBU促进邻氯甲基苯胺与with内酯的正式[4 + 2]环化反应
更新日期:2020-11-03
Synthesis ( IF 2.2 ) Pub Date : 2020-11-02 , DOI: 10.1055/s-0040-1706549 Jianfeng Xu 1 , Haojie Ji 1 , Chonglong He 1 , Hongjie Gao 2 , Weijun Fu 3
Affiliation
|
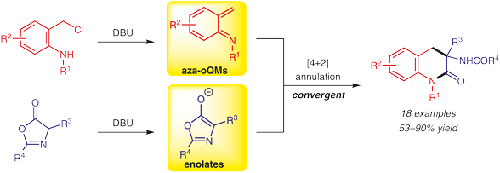
An efficient 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU)-mediated formal [4+2] annulation reaction of aza-ortho-quinone methides (aza-oQMs) (generated from o-chloromethyl anilines) with enolates (formed from azlactones) is disclosed, delivering biologically significant 3,4-dihydroquinolin-2(1H)-one derivatives in moderate to good yields. The salient features of this reaction include readily accessible starting materials, broad substrate scope, mild reaction conditions, removable protecting groups, and a scalable synthetic approach.
中文翻译:

DBU促进邻氯甲基苯胺与with内酯的正式[4 + 2]环化反应
有效的1,8-二氮杂双环[5.4.0]十一碳-7-烯(DBU)介导的氮杂-邻-醌甲基化物(aza-oQMs)(由邻氯甲基苯胺生成)的正式[4 + 2]成环反应公开了具有烯醇盐(由氮杂内酯形成)的α-内酯,以中等至良好的产率递送生物学上重要的3,4-二氢喹啉-2(1H)-一衍生物。该反应的显着特征包括容易获得的起始原料,广泛的底物范围,温和的反应条件,可去除的保护基团和可扩展的合成方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号