当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stepwise Suzuki−Miyaura Cross‐Coupling of Triborylalkenes Derived from Alkynyl−B(dan)s: Regioselective and Flexible Synthesis of Tetrasubstituted Alkenes
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-11-02 , DOI: 10.1002/adsc.202001116 Tomohiro Tani 1 , Naomi Takahashi 1 , Yuuki Sawatsugawa 1 , Mana Osano 1 , Teruhisa Tsuchimoto 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-11-02 , DOI: 10.1002/adsc.202001116 Tomohiro Tani 1 , Naomi Takahashi 1 , Yuuki Sawatsugawa 1 , Mana Osano 1 , Teruhisa Tsuchimoto 1
Affiliation
|
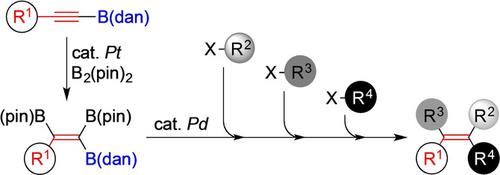
Alkenes with three boryl groups of differing reactivities were synthesized and subsequently cross‐coupled regioselectively with aryl halides in a stepwise manner to afford tetrasubstituted alkenes. The key triborylalkene is derived from the platinum‐catalyzed diboration of alkynyl−B(dan)s with B2(pin)2. Due to excellent regioselectivity and reaction efficiency of each step starting with alkynyl−B(dan)s, tetrasubstituted alkenes with desired carbon frameworks at desired positions can be prepared in high yields. For example, an alkene with four distinct aryl groups, p‐MeOC6H4, p‐CF3C6H4, p‐MeC6H4, and p‐NCC6H4, was obtained in 71% overall yield via six steps, starting with the dehydrogenative borylation of p‐MeC6H4C≡CH with HB(dan). Moreover, a variety of tetrasubstituted alkenes, including regio‐ and stereoisomers of the above tetraarylalkene, AIE‐active TPTPE and derivatives thereof, and (Z)‐tamoxifen, a well‐known breast cancer drug, were accessed via the developed strategy.
中文翻译:

烷基-B(dan)s衍生的三硼烷基烯烃的逐步Suzuki-Miyaura交叉偶联:四取代烯烃的区域选择性和柔性合成
合成了具有三个不同反应性的硼烷基的烯烃,然后逐步与芳基卤化物进行区域选择性交联,得到四取代的烯烃。关键的三硼烷基烯烃衍生自乙炔基B(dan)s与B 2(pin)2的铂催化二硼酸酯化反应。由于从炔基-B(dan)s开始的每个步骤都具有出色的区域选择性和反应效率,因此可以高收率制备在所需位置具有所需碳骨架的四取代烯烃。例如,具有四个不同芳基的烯烃,p- MeOC 6 H 4,p- CF 3 C 6 H 4,p-MeC 6 ħ 4,和p -NCC 6 ħ 4,经六个步骤以71%的总产率获得,先从的脱氢硼化p -MeC 6 ħ 4 C≡CH与HB(旦)。此外,通过制定的策略可以使用多种四取代烯烃,包括上述四芳基烯烃的区域和立体异构体,AIE活性TPTPE及其衍生物,以及著名的乳腺癌药物(Z)-他莫昔芬。
更新日期:2020-11-02
中文翻译:

烷基-B(dan)s衍生的三硼烷基烯烃的逐步Suzuki-Miyaura交叉偶联:四取代烯烃的区域选择性和柔性合成
合成了具有三个不同反应性的硼烷基的烯烃,然后逐步与芳基卤化物进行区域选择性交联,得到四取代的烯烃。关键的三硼烷基烯烃衍生自乙炔基B(dan)s与B 2(pin)2的铂催化二硼酸酯化反应。由于从炔基-B(dan)s开始的每个步骤都具有出色的区域选择性和反应效率,因此可以高收率制备在所需位置具有所需碳骨架的四取代烯烃。例如,具有四个不同芳基的烯烃,p- MeOC 6 H 4,p- CF 3 C 6 H 4,p-MeC 6 ħ 4,和p -NCC 6 ħ 4,经六个步骤以71%的总产率获得,先从的脱氢硼化p -MeC 6 ħ 4 C≡CH与HB(旦)。此外,通过制定的策略可以使用多种四取代烯烃,包括上述四芳基烯烃的区域和立体异构体,AIE活性TPTPE及其衍生物,以及著名的乳腺癌药物(Z)-他莫昔芬。











































 京公网安备 11010802027423号
京公网安备 11010802027423号