当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and biochemical characterizations of the novel autolysin Acd24020 from Clostridioides difficile and its full‐function catalytic domain as a lytic enzyme
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-11-02 , DOI: 10.1111/mmi.14636 Hiroshi Sekiya 1 , Eiji Tamai 1, 2 , Jurina Kawasaki 1 , Kaho Murakami 1 , Shigehiro Kamitori 2
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-11-02 , DOI: 10.1111/mmi.14636 Hiroshi Sekiya 1 , Eiji Tamai 1, 2 , Jurina Kawasaki 1 , Kaho Murakami 1 , Shigehiro Kamitori 2
Affiliation
|
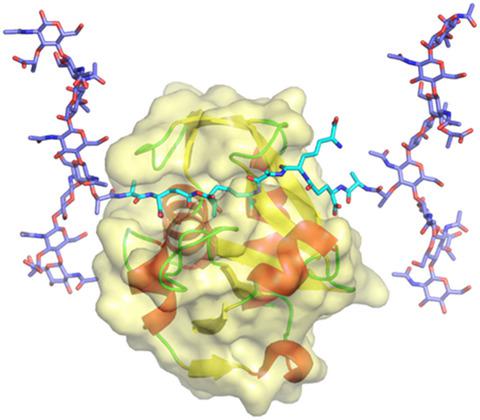
Autolysin is a lytic enzyme that hydrolyzes peptidoglycans of the bacterial cell wall, with a catalytic domain and cell wall‐binding (CWB) domains, to be involved in different physiological functions that require bacterial cell wall remodeling. We identified a novel autolysin, Acd24020, from Clostridioides (Clostridium) difficile (C. difficile), with an endopeptidase catalytic domain belonging to the NlpC/P60 family and three bacterial Src‐homology 3 domains as CWB domains. The catalytic domain of Acd24020 (Acd24020‐CD) exhibited C. difficile‐specific lytic activity equivalent to Acd24020, indicating that Acd24020‐CD has full‐function as a lytic enzyme by itself. To elucidate the specific peptidoglycan‐recognition and catalytic reaction mechanisms of Acd24020‐CD, biochemical characterization, X‐ray structure determination, a modeling study of the enzyme/substrate complex, and mutagenesis analysis were performed. Acd24020‐CD has an hourglass‐shaped substrate‐binding groove across the molecule, which is responsible for recognizing the direct 3–4 cross‐linking structure unique to C. difficile peptidoglycan. Based on the X‐ray structure and modeling study, we propose a dynamic Cys/His catalyzing mechanism, in which the catalytic Cys299 and His354 residues dynamically change their conformations to complement each step of the enzyme catalytic reaction.
中文翻译:

来自艰难梭菌的新型自溶素 Acd24020 的结构和生化特征及其作为裂解酶的全功能催化域
自溶素是一种裂解酶,可水解细菌细胞壁的肽聚糖,具有催化域和细胞壁结合 (CWB) 域,参与需要细菌细胞壁重塑的不同生理功能。我们鉴定了新的自溶素,Acd24020,从Clostridioides(梭菌)艰难(艰难梭菌)中,用内肽酶催化结构域属于NLPC / P60家庭和三个细菌的Src同源性3个结构域CWB域。Acd24020 (Acd24020-CD) 的催化域表现出艰难梭菌-特异性裂解活性相当于 Acd24020,表明 Acd24020-CD 本身具有完整的裂解酶功能。为了阐明 Acd24020-CD 的特定肽聚糖识别和催化反应机制,进行了生化表征、X 射线结构测定、酶/底物复合物的建模研究和诱变分析。Acd24020-CD 在分子上有一个沙漏形的底物结合槽,负责识别艰难梭菌肽聚糖特有的直接 3-4 交联结构。基于X射线结构和建模研究,我们提出了一种动态Cys/His催化机制,其中催化性Cys299和His354残基动态改变其构象以补充酶催化反应的每一步。
更新日期:2020-11-02
中文翻译:

来自艰难梭菌的新型自溶素 Acd24020 的结构和生化特征及其作为裂解酶的全功能催化域
自溶素是一种裂解酶,可水解细菌细胞壁的肽聚糖,具有催化域和细胞壁结合 (CWB) 域,参与需要细菌细胞壁重塑的不同生理功能。我们鉴定了新的自溶素,Acd24020,从Clostridioides(梭菌)艰难(艰难梭菌)中,用内肽酶催化结构域属于NLPC / P60家庭和三个细菌的Src同源性3个结构域CWB域。Acd24020 (Acd24020-CD) 的催化域表现出艰难梭菌-特异性裂解活性相当于 Acd24020,表明 Acd24020-CD 本身具有完整的裂解酶功能。为了阐明 Acd24020-CD 的特定肽聚糖识别和催化反应机制,进行了生化表征、X 射线结构测定、酶/底物复合物的建模研究和诱变分析。Acd24020-CD 在分子上有一个沙漏形的底物结合槽,负责识别艰难梭菌肽聚糖特有的直接 3-4 交联结构。基于X射线结构和建模研究,我们提出了一种动态Cys/His催化机制,其中催化性Cys299和His354残基动态改变其构象以补充酶催化反应的每一步。











































 京公网安备 11010802027423号
京公网安备 11010802027423号