当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hemeprotein Tpx1 interacts with cell‐surface heme transporter Str3 in Schizosaccharomyces pombe
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-11-02 , DOI: 10.1111/mmi.14638 Vincent Normant 1 , Ariane Brault 1 , Mariano Avino 1 , Thierry Mourer 1 , Tobias Vahsen 1 , Jude Beaudoin 1 , Simon Labbé 1
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-11-02 , DOI: 10.1111/mmi.14638 Vincent Normant 1 , Ariane Brault 1 , Mariano Avino 1 , Thierry Mourer 1 , Tobias Vahsen 1 , Jude Beaudoin 1 , Simon Labbé 1
Affiliation
|
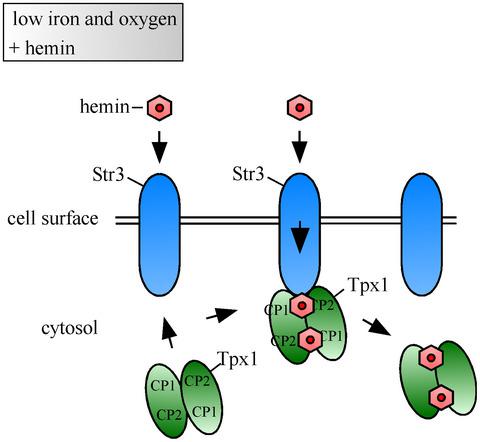
Str3 is a transmembrane protein that mediates low‐affinity heme uptake in Schizosaccharomyces pombe. Under iron‐limiting conditions, Str3 remains at the cell surface in the presence of increasing hemin concentrations. Using a proximity‐dependent biotinylation approach coupled to mass spectrometry and coimmunoprecipitation assays, we report that the peroxiredoxin Tpx1 is a binding partner of Str3. Under microaerobic conditions, cells deficient in heme biosynthesis and lacking the heme receptor Shu1 exhibit poor hemin‐dependent growth in the absence of Tpx1. Analysis of membrane protein preparations from iron‐starved hem1Δ shu1Δ str3Δ tpx1Δ cells coexpressing Str3‐GFP and TAP‐Tpx1 showed that TAP‐Tpx1 is enriched in membrane protein fractions in response to hemin. Bimolecular fluorescence complementation assays brought additional evidence that an interaction between Tpx1 and Str3 occurs at the plasma membrane. Results showed that Tpx1 exhibits an equilibrium constant value of 0.26 μM for hemin. The association of Tpx1 with hemin protects hemin from degradation by H2O2. The peroxidase activity of hemin is lowered when it is bound to Tpx1. Taken together, these results revealed that Tpx1 is a novel interacting partner of Str3. Our data are the first example of an interaction between a cytoplasmic heme‐binding protein and a cell‐surface heme transporter.
中文翻译:

血红素蛋白 Tpx1 与粟酒裂殖酵母细胞表面血红素转运蛋白 Str3 相互作用
Str3 是一种跨膜蛋白,可介导粟酒裂殖酵母中的低亲和力血红素摄取。在铁限制条件下,在血红素浓度增加的情况下,Str3 保留在细胞表面。使用邻近依赖性生物素化方法与质谱和共免疫沉淀分析相结合,我们报告说过氧还蛋白 Tpx1 是 Str3 的结合伴侣。在微需氧条件下,缺乏血红素生物合成和缺乏血红素受体 Shu1 的细胞在缺乏 Tpx1 的情况下表现出较差的血红素依赖性生长。铁缺乏hem1Δ shu1Δ str3Δ tpx1Δ膜蛋白制剂的分析共表达 Str3-GFP 和 TAP-Tpx1 的细胞表明,TAP-Tpx1 富含膜蛋白组分以响应血红素。双分子荧光互补分析提供了额外的证据,表明 Tpx1 和 Str3 之间的相互作用发生在质膜上。结果表明,Tpx1 的血红素平衡常数值为 0.26 μM。Tpx1 与氯化血红素的结合保护氯化血红素免于被 H 2 O 2降解。与 Tpx1 结合后,血红素的过氧化物酶活性降低。综上所述,这些结果表明 Tpx1 是 Str3 的新型相互作用伙伴。我们的数据是细胞质血红素结合蛋白和细胞表面血红素转运蛋白相互作用的第一个例子。
更新日期:2020-11-02
中文翻译:

血红素蛋白 Tpx1 与粟酒裂殖酵母细胞表面血红素转运蛋白 Str3 相互作用
Str3 是一种跨膜蛋白,可介导粟酒裂殖酵母中的低亲和力血红素摄取。在铁限制条件下,在血红素浓度增加的情况下,Str3 保留在细胞表面。使用邻近依赖性生物素化方法与质谱和共免疫沉淀分析相结合,我们报告说过氧还蛋白 Tpx1 是 Str3 的结合伴侣。在微需氧条件下,缺乏血红素生物合成和缺乏血红素受体 Shu1 的细胞在缺乏 Tpx1 的情况下表现出较差的血红素依赖性生长。铁缺乏hem1Δ shu1Δ str3Δ tpx1Δ膜蛋白制剂的分析共表达 Str3-GFP 和 TAP-Tpx1 的细胞表明,TAP-Tpx1 富含膜蛋白组分以响应血红素。双分子荧光互补分析提供了额外的证据,表明 Tpx1 和 Str3 之间的相互作用发生在质膜上。结果表明,Tpx1 的血红素平衡常数值为 0.26 μM。Tpx1 与氯化血红素的结合保护氯化血红素免于被 H 2 O 2降解。与 Tpx1 结合后,血红素的过氧化物酶活性降低。综上所述,这些结果表明 Tpx1 是 Str3 的新型相互作用伙伴。我们的数据是细胞质血红素结合蛋白和细胞表面血红素转运蛋白相互作用的第一个例子。









































 京公网安备 11010802027423号
京公网安备 11010802027423号