当前位置:
X-MOL 学术
›
Luminescence
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Study on the interaction of hyperoside and human serum albumin in VC and VC‐free environments by spectroscopic and molecular docking techniques
Luminescence ( IF 3.2 ) Pub Date : 2020-11-03 , DOI: 10.1002/bio.3978 Xiulan Xin 1 , Liang Chen 1 , Ye Li 1 , Ran Yu 1 , Haitao Fan 1 , Zheng Yan 1 , Shuangshi Li 1 , Hui Feng 1
Luminescence ( IF 3.2 ) Pub Date : 2020-11-03 , DOI: 10.1002/bio.3978 Xiulan Xin 1 , Liang Chen 1 , Ye Li 1 , Ran Yu 1 , Haitao Fan 1 , Zheng Yan 1 , Shuangshi Li 1 , Hui Feng 1
Affiliation
|
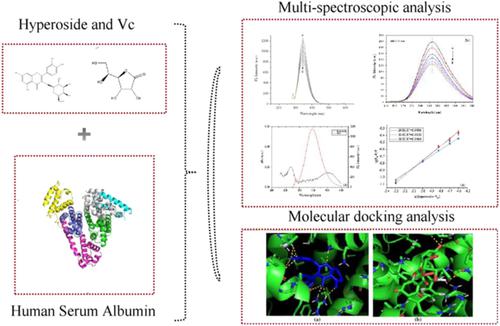
The interaction between hyperoside and human serum albumin was studied in vitamin C (VC) and VC‐free environments using ultraviolet (UV)–vis absorption, fluorescence, circular dichroism spectra, and molecular docking techniques under simulated physiological conditions. The two environments had different influences on the secondary structure of human serum albumin (HSA). The α‐helix content was slightly increased from 50% to 51% in the VC environment and increased from 50% to 55% in the VC‐free environment. The thermodynamic parameters were ΔH° = −30.7 kJ⋅mol−1 and ΔS° = −23.4 mol−1⋅K−1 in the VC environment and ΔH° = −25.4 kJ⋅mol−1 and ΔS° = −11.4 J⋅mol−1⋅K−1 in the VC‐free environment. Through thermodynamics parameters, hydrophobic force played a dominant role in the whole environment. The binding constants were calculated to be 7.25 × 105 mol⋅L−1 and 9.76 × 105 mol⋅L−1 at 298 K and they declined with the rise in temperature. The two binding distances were 2.6 nm and 2.5 nm respectively at 298 K, indicating that fluorescence energy transfer occurred. The UV–vis spectra indicated that fluorescence quenching of the HSA–hyperoside complex was a static quenching process. Hyperoside could spontaneously bind to HSA at site I (subdomain IIA). Molecular docking elucidated the way to binding basically through hydrophobic and van der Waals force interactions. Moreover, molecular docking showed that the VC environment could influence binding of HSA and hyperoside by more H‐binding and less hydrophobic forces.
中文翻译:

通过光谱和分子对接技术研究VC和无VC环境中超高苷与人血清白蛋白的相互作用
在维生素C(V C)和无V C的环境中,使用紫外(UV)-vis吸收,荧光,圆二色性光谱和分子对接技术,在模拟的生理条件下,研究了Hyperoside与人血清白蛋白之间的相互作用。两种环境对人血清白蛋白(HSA)二级结构的影响不同。的α螺旋含量略微从50%增加至51%在V Ç环境和在V从50%提高到55%Ç -free环境。的热力学参数是Δ ħ °= -30.7kJ⋅mol -1和Δ小号°= -23.4摩尔-1 ⋅K -1在V Ç环境和Δ ħ °= -25.4kJ⋅mol -1和Δ小号°= -11.4J⋅mol -1 ⋅K -1在V Ç -free环境。通过热力学参数,疏水力在整个环境中起着主导作用。的结合常数分别计算为7.25×10 5 mol⋅L -1和9.76×10 5 mol⋅L -1在298 K时,它们随着温度的升高而下降。在298 K时,两个结合距离分别为2.6 nm和2.5 nm,表明发生了荧光能量转移。紫外-可见光谱表明,HSA-高糖苷复合物的荧光猝灭是一个静态的猝灭过程。金丝桃苷可以在位点I(亚结构域IIA)上自发地与HSA结合。分子对接阐明了基本上通过疏水力和范德华力相互作用的结合方式。此外,分子对接表明,V C环境可通过更多的H结合和较小的疏水力影响HSA和超高苷的结合。
更新日期:2020-11-03
中文翻译:

通过光谱和分子对接技术研究VC和无VC环境中超高苷与人血清白蛋白的相互作用
在维生素C(V C)和无V C的环境中,使用紫外(UV)-vis吸收,荧光,圆二色性光谱和分子对接技术,在模拟的生理条件下,研究了Hyperoside与人血清白蛋白之间的相互作用。两种环境对人血清白蛋白(HSA)二级结构的影响不同。的α螺旋含量略微从50%增加至51%在V Ç环境和在V从50%提高到55%Ç -free环境。的热力学参数是Δ ħ °= -30.7kJ⋅mol -1和Δ小号°= -23.4摩尔-1 ⋅K -1在V Ç环境和Δ ħ °= -25.4kJ⋅mol -1和Δ小号°= -11.4J⋅mol -1 ⋅K -1在V Ç -free环境。通过热力学参数,疏水力在整个环境中起着主导作用。的结合常数分别计算为7.25×10 5 mol⋅L -1和9.76×10 5 mol⋅L -1在298 K时,它们随着温度的升高而下降。在298 K时,两个结合距离分别为2.6 nm和2.5 nm,表明发生了荧光能量转移。紫外-可见光谱表明,HSA-高糖苷复合物的荧光猝灭是一个静态的猝灭过程。金丝桃苷可以在位点I(亚结构域IIA)上自发地与HSA结合。分子对接阐明了基本上通过疏水力和范德华力相互作用的结合方式。此外,分子对接表明,V C环境可通过更多的H结合和较小的疏水力影响HSA和超高苷的结合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号