当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interplay between Structure and Mechanism in Reductive Dissociative Electron Transfers to α,β ‐Epoxyketones
ChemPlusChem ( IF 3.0 ) Pub Date : 2020-11-03 , DOI: 10.1002/cplu.202000305 Jared N. Spencer 1 , Michelle L. Grimm 2 , James M. Tanko 2
ChemPlusChem ( IF 3.0 ) Pub Date : 2020-11-03 , DOI: 10.1002/cplu.202000305 Jared N. Spencer 1 , Michelle L. Grimm 2 , James M. Tanko 2
Affiliation
|
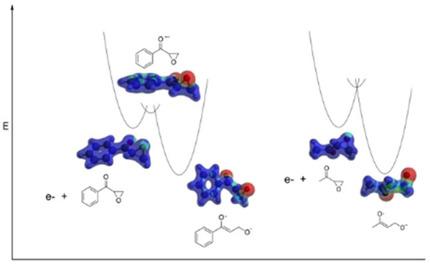
The electrochemical reduction of several α,β ‐epoxyketones was studied using cyclic (linear sweep) voltammetry, convolution voltammetry, and homogeneous redox catalysis. The results were reconciled to pertinent theories of electron transfer. α,β ‐Epoxyketones undergo dissociative electron‐transfer reactions with C−O bond cleavage, via both stepwise and concerted mechanisms, depending on their structure. For aliphatic ketones, the preferred mechanism of reduction is consistent with the “sticky” concerted model for dissociative electron transfer. Bond cleavage occurs simultaneously with electron transfer, and there is a residual, electrostatic interaction in the ring‐opened (distonic) radical anion. In contrast, for aromatic ketones, because the ring‐closed radical anions are resonance‐stabilized and exist at energy minima, a stepwise mechanism operates (electron transfer and bond cleavage occur in discrete steps). The rate constants for ring opening are on the order of 108 s−1, and not significantly affected by substituents on the 3‐membered ring (consistent with C−O bond cleavage). These results and conclusions were fully supported and augmented by molecular orbital calculations.
中文翻译:

还原解离电子转移至α,β-环氧酮的结构与机理之间的相互作用
使用循环(线性扫描)伏安法,卷积伏安法和均相氧化还原催化研究了几种α,β-环氧酮的电化学还原。结果与相关的电子转移理论相符。α,β-环氧酮根据结构的不同,通过逐步和协同机理经历具有C-O键断裂的解离电子转移反应。对于脂肪族酮,优选的还原机理与用于解离电子转移的“粘性”协同模型一致。键裂解与电子转移同时发生,并且在开环(张力)自由基阴离子中存在残留的静电相互作用。相反,对于芳族酮,由于闭环自由基阴离子是共振稳定的,并且以最小能量存在,逐步机制起作用(电子转移和键裂解发生在不连续的步骤中)。开环的速率常数约为108 s -1,并且不受三元环上取代基的显着影响(与C-O键断裂一致)。这些结果和结论得到分子轨道计算的充分支持和扩充。
更新日期:2020-11-03
中文翻译:

还原解离电子转移至α,β-环氧酮的结构与机理之间的相互作用
使用循环(线性扫描)伏安法,卷积伏安法和均相氧化还原催化研究了几种α,β-环氧酮的电化学还原。结果与相关的电子转移理论相符。α,β-环氧酮根据结构的不同,通过逐步和协同机理经历具有C-O键断裂的解离电子转移反应。对于脂肪族酮,优选的还原机理与用于解离电子转移的“粘性”协同模型一致。键裂解与电子转移同时发生,并且在开环(张力)自由基阴离子中存在残留的静电相互作用。相反,对于芳族酮,由于闭环自由基阴离子是共振稳定的,并且以最小能量存在,逐步机制起作用(电子转移和键裂解发生在不连续的步骤中)。开环的速率常数约为108 s -1,并且不受三元环上取代基的显着影响(与C-O键断裂一致)。这些结果和结论得到分子轨道计算的充分支持和扩充。











































 京公网安备 11010802027423号
京公网安备 11010802027423号