当前位置:
X-MOL 学术
›
Chemistryopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tyrosine Side‐Chain Functionalities at Distinct Positions Determine the Chirooptical Properties and Supramolecular Structures of Pentameric Oligothiophenes
ChemistryOpen ( IF 2.5 ) Pub Date : 2020-11-03 , DOI: 10.1002/open.202000144 Marcus Bäck 1 , Robert Selegård 2 , Yogesh Todarwal 3 , Sofie Nyström 1 , Patrick Norman 3 , Mathieu Linares 4, 5, 6 , Per Hammarström 1 , Mikael Lindgren 1, 7 , K Peter R Nilsson 1
ChemistryOpen ( IF 2.5 ) Pub Date : 2020-11-03 , DOI: 10.1002/open.202000144 Marcus Bäck 1 , Robert Selegård 2 , Yogesh Todarwal 3 , Sofie Nyström 1 , Patrick Norman 3 , Mathieu Linares 4, 5, 6 , Per Hammarström 1 , Mikael Lindgren 1, 7 , K Peter R Nilsson 1
Affiliation
|
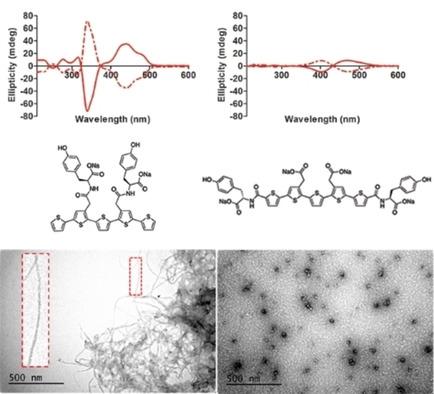
Control over the photophysical properties and molecular organization of π‐conjugated oligothiophenes is essential to their use in organic electronics. Herein we synthesized and characterized a variety of anionic pentameric oligothiophenes with different substitution patterns of L‐ or D‐tyrosine at distinct positions along the thiophene backbone. Spectroscopic, microscopic, and theoretical studies of L‐ or D‐tyrosine substituted pentameric oligothiophene conjugates revealed the formation of optically active π‐stacked self‐assembled aggregates under acid conditions. The distinct photophysical characteristics, as well as the supramolecular structures of the assemblies, were highly influenced by the positioning of the L‐ or D‐tyrosine moieties along the thiophene backbone. Overall, the obtained results clearly demonstrate how fundamental changes in the position of the enantiomeric side‐chain functionalities greatly affect the optical properties as well as the architecture of the self‐assembled supramolecular structures.
中文翻译:

不同位置的酪氨酸侧链官能团决定五聚低聚噻吩的手性光学性质和超分子结构
控制π共轭低聚噻吩的光物理性质和分子组织对于其在有机电子学中的应用至关重要。在此,我们合成并表征了多种阴离子五聚寡聚噻吩,在噻吩主链的不同位置上具有不同的 L-或 D-酪氨酸取代模式。 L-或D-酪氨酸取代的五聚寡聚噻吩缀合物的光谱、显微镜和理论研究揭示了在酸性条件下形成光学活性π堆叠自组装聚集体。不同的光物理特性以及组件的超分子结构很大程度上受到沿噻吩主链的 L-或 D-酪氨酸部分的定位的影响。总的来说,所获得的结果清楚地证明了对映体侧链官能团位置的根本变化如何极大地影响光学性质以及自组装超分子结构的结构。
更新日期:2020-11-03
中文翻译:

不同位置的酪氨酸侧链官能团决定五聚低聚噻吩的手性光学性质和超分子结构
控制π共轭低聚噻吩的光物理性质和分子组织对于其在有机电子学中的应用至关重要。在此,我们合成并表征了多种阴离子五聚寡聚噻吩,在噻吩主链的不同位置上具有不同的 L-或 D-酪氨酸取代模式。 L-或D-酪氨酸取代的五聚寡聚噻吩缀合物的光谱、显微镜和理论研究揭示了在酸性条件下形成光学活性π堆叠自组装聚集体。不同的光物理特性以及组件的超分子结构很大程度上受到沿噻吩主链的 L-或 D-酪氨酸部分的定位的影响。总的来说,所获得的结果清楚地证明了对映体侧链官能团位置的根本变化如何极大地影响光学性质以及自组装超分子结构的结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号