Results in Chemistry ( IF 2.5 ) Pub Date : 2019-12-05 , DOI: 10.1016/j.rechem.2019.100019 Hilde V. Halleraker , Solmaz Ghoreishi , Tanja Barth
|
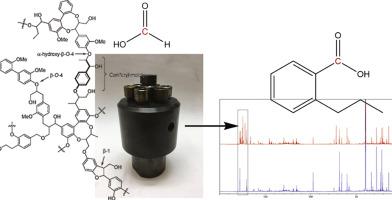
The lignin to liquid (LtL) solvolysis is a type of hydrothermal liquefaction (HTL) that utilizes formic acid to facilitate the desired hydrodeoxygenation process to depolymerize lignin. Formic acid has been chosen due to its hydrogen donor ability, and it has proven to be a more efficient hydrogen donor than hydrogen gas and isopropanol. Based on previous experiments where the amount of carbon in the solid phase and organic phase together exceeded 100% relative to carbon in the lignin starting material, we set up experiments with 13C-labeled formic acid in order to investigate where the carbon form formic acid is incorporated. All the experiments were performed in duplicates, one with 13C-labeled formic acid and one with standard formic acid. The bio-oils from the experiments were analyzed by Nuclear Magnetic Resonance (NMR) and the spectra were compared. The 13C NMR spectra showed significant differences between oils made with standard formic acid and oils made with 13C-labeled formic acid, located in the section between δ 170 ppm and δ 180 ppm in the NMR spectra. The signal in this range arise from carbonyl bonds and shows that the carbon from formic acid ends up in the carbonyl structures of the bio-oil in compounds such as aromatic acids. This paper has also shown that the amount of formic acid plays a minor role in product composition compared to reaction temperature, which influences the product composition on a functional group level.
中文翻译:

使用13 C标记的甲酸和13 C NMR研究HTL条件下甲酸和木质素的反应途径
木质素到液体(LtL)的溶剂分解是一种水热液化(HTL),它利用甲酸来促进所需的加氢脱氧过程以解聚木质素。选择甲酸是因为它具有氢供体的能力,并且事实证明它是比氢气和异丙醇更有效的氢供体。根据先前的实验,其中固相和有机相中的碳含量相对于木质素原料中的碳含量超过100%,我们建立了使用13 C标记的甲酸的实验,以研究碳形成甲酸的位置成立。所有实验均一式两份进行,一式三份C标记的甲酸和一种带有标准甲酸的甲酸。通过核磁共振(NMR)分析了来自实验的生物油,并比较了光谱。的13 C NMR谱显示出与标准甲酸和油与由制成油之间显著差异13 C标记的甲酸,位于所述部分之间δ 170 ppm或δNMR光谱中为180 ppm。此范围内的信号来自羰基键,表明来自甲酸的碳最终进入生物油的芳族酸等化合物的羰基结构中。本文还表明,与反应温度相比,甲酸的量在产物组成中的作用较小,这会在官能团水平上影响产物的组成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号