Results in Chemistry ( IF 2.5 ) Pub Date : 2020-04-02 , DOI: 10.1016/j.rechem.2020.100037 Bhaskar Chakraborty , Esmita Chettri
|
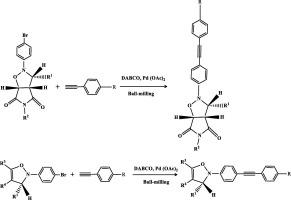
A greener strategy for the synthesis of some functionalized heterocycles have been developed using Sonogashira cross-coupling reaction of furfural derived isoxazolidine and isoxazoline derivatives. This is a new approach for the functionalization of isoxazolidine and isoxazoline derivatives to modified heterocycles via cross-coupling reaction in solvent free ball-milling process. Since this kind of functionalization of cycloadducts to modified heterocycles via cross-coupling reaction are not known in literature, therefore, this approach will surely attract synthetic chemists to plan for this kind of reactions in organic synthesis. Significant anticancer activity of the new molecules added extra flavour in this synthesis. Simple reaction methodology, good to excellent yields and greener approaches are the important features noticed in this syntheses.
中文翻译:

Sonogashira交叉偶联反应机械合成某些杂环分子及其抗癌活性
使用糠醛衍生的异恶唑烷和异恶唑啉衍生物的Sonogashira交叉偶联反应,已经开发出一种更绿色的合成某些官能化杂环的策略。这是在无溶剂球磨过程中通过交叉偶联反应将异恶唑烷和异恶唑啉衍生物官能化为改性杂环的新方法。由于在文献中还不知道这种通过环偶联反应将环加合物官能化为修饰的杂环的方法,因此,这种方法必将吸引合成化学家为有机合成中的这类反应做计划。新分子的显着抗癌活性在这种合成中增加了额外的风味。简单的反应方法,











































 京公网安备 11010802027423号
京公网安备 11010802027423号