Results in Chemistry ( IF 2.5 ) Pub Date : 2020-07-21 , DOI: 10.1016/j.rechem.2020.100064 Dhruba Jyoti Boruah , Panneerselvam Yuvaraj , Ram Awatar Maurya
|
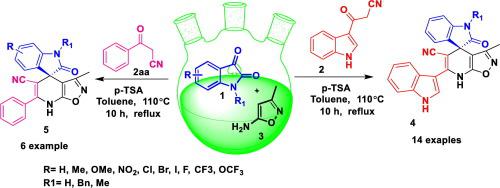
With their exceptional three-dimensional structural topographies, spirooxindoles are known best for privileged chemotypes for diverse biological applications. We report herin a highly convergent and efficient protocol, for the facile chemoselective synthesis of a library of [indoline-3,4′-isoxazolo[5,4-b]pyridine fused spirooxindole derivatives, has been achieved by a Brønsted acid catalyzed three component tandem Knoevenagel/Michael addition. Interestingly, the method not only offers the benefits of operational simplicity, but also chemoselective and atom economic with excellent yields of the targeted molecule. The reaction mechanism and substrate scope of this novel reaction has been thoroughly out lined.
中文翻译:

[二氢吲哚-3,4'-异恶唑的化学-选择性合成并[5,4- b ]吡啶稠合的衍生物spirooxindole通过布朗斯台德酸催化三组分串联的Knoevenagel / Michael加成反应
螺硫辛多凭借其独特的三维结构形貌,以其在多种生物应用中的优先化学型而著称。我们报告herin一个高度收敛和有效的协议,为[吲哚啉-3,4'-异恶唑并[5,4- b ]吡啶稠合的螺氧杂吲哚衍生物的文库的轻松化学选择性合成,已通过布朗斯台德酸催化的三组分实现串联Knoevenagel / Michael。有趣的是,该方法不仅具有操作简便的优势,而且还具有化学选择性和原子经济性,并具有优异的目标分子收率。该新颖反应的反应机理和底物范围已被彻底勾画出来。











































 京公网安备 11010802027423号
京公网安备 11010802027423号