Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-11-03 , DOI: 10.1016/j.cej.2020.127593 Shenghui Yu , Cheng Zhang , Lun Ma , Qingyan Fang , Gang Chen
|
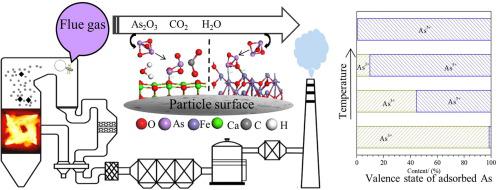
As a highly toxic element of coal, arsenic (As) is mainly converted into As2O3 vapor during coal combustion. Mineral oxides are effective and abundant sorbents to control As2O3 emissions. In this paper, the characteristics of As2O3 adsorption by mineral oxides were studied in a two-stage reactor, and the density of states, adsorption sites, and adsorption energies for the adsorption reaction were investigated using molecular simulation. The results demonstrate that CaO is an excellent As2O3 sorbent. The As2O3 adsorption effect of CaO, MgO, and Al2O3 was found to become better with increasing temperature within the range of 300-900 ℃. The order of As2O3 adsorption at 300-700 ℃ follows CaO > Fe2O3 > Na2O > MgO > Al2O3 > SiO2, and SiO2 hardly adsorbs any As2O3. At 900 ℃, the melting of Na2O and Fe2O3 greatly reduces their As2O3 adsorption capacity. The O atoms in Na2O/CaO/MgO/Al2O3/SiO2 and the Fe atoms in Fe2O3 are the adsorption active sites for As2O3. The order of the calculated adsorption energy follows Fe2O3 > Na2O > CaO > Al2O3 > MgO > SiO2, and the As2O3 adsorption involves chemical adsorption. The adsorption products are Ca3(AsO4)2, FeAsO4, AlAsO4, etc., for which As exists in the form of As3+ and As5+, with As3+ transformed into As5+ with increasing adsorption temperature.
中文翻译:

矿物氧化物吸附剂对As 2 O 3吸附特性的认识:实验和DFT研究
作为煤的剧毒元素,砷在煤燃烧过程中主要转化为As 2 O 3蒸气。矿物氧化物是有效和丰富的吸附剂,可控制As 2 O 3的排放。在两级反应器中研究了矿物氧化物对As 2 O 3的吸附特性,并通过分子模拟研究了吸附反应的态密度,吸附位和吸附能。结果表明,CaO是优良的As 2 O 3吸附剂。CaO,MgO和Al 2 O对As 2 O 3的吸附作用发现在300-900℃范围内,随着温度的升高3变好。在300-700℃时As 2 O 3的吸附顺序为CaO> Fe 2 O 3 > Na 2 O> MgO> Al 2 O 3 > SiO 2,SiO 2几乎不吸附任何As 2 O 3。在900℃时,Na 2 O和Fe 2 O 3的熔融大大降低了其对As 2 O 3的吸附能力。Na 2 O / CaO / MgO / Al 2 O 3中的O原子/ SiO 2和Fe 2 O 3中的Fe原子是As 2 O 3的吸附活性位。计算出的吸附能量的顺序为Fe 2 O 3 > Na 2 O> CaO> Al 2 O 3 > MgO> SiO 2,而As 2 O 3的吸附涉及化学吸附。吸附产物为Ca 3(AsO 4)2,FeAsO 4,AlAsO 4等,其中As以As 3+和As 5+的形式存在,随着吸附温度的升高,As 3+转化为As 5+。











































 京公网安备 11010802027423号
京公网安备 11010802027423号