当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improved batch and flow syntheses of the nonsteroidal anti-inflammatory COX-2 inhibitor celecoxib
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2020-10-20 , DOI: 10.1039/d0re00346h Chantal Scholtz 1, 2, 3, 4 , Darren L. Riley 1, 2, 3, 4
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2020-10-20 , DOI: 10.1039/d0re00346h Chantal Scholtz 1, 2, 3, 4 , Darren L. Riley 1, 2, 3, 4
Affiliation
|
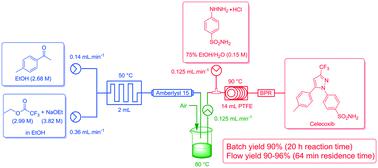
The comparison of an improved conventional batch mode synthesis of the nonsteroidal anti-inflammatory COX-2 inhibitor celecoxib with its flow chemistry alternative is reported. The stepwise and continuous flow synthesis of celecoxib was achieved by means of a Claisen condensation to access 4,4,4-trifluoro-1-(4-methyl-phenyl)-butane-1,3-dione followed by a cyclo-condensation reaction with 4-sulfamidophenylhydrazine hydrochloride to obtain the pyrazole moiety. A batch process was developed with improved work-up and purification (90% yield). This was successfully translated to flow in yields of 90–96% with greatly shortened reaction times (20 h vs. 1 h) and reduced chemical exposure.
中文翻译:

非甾体类抗炎COX-2抑制剂塞来昔布的批次和流动合成的改进
据报道,对非甾体抗炎COX-2抑制剂塞来昔布的改进的常规分批模式合成及其流动化学选择进行了比较。塞来昔布的逐步连续流动合成是通过克莱森缩合反应获得4,4,4-三氟-1-(4-甲基-苯基)-丁烷-1,3-二酮,然后进行环缩合反应而实现的用4-磺酰胺基苯肼盐酸盐得到吡唑部分。开发了具有改进的后处理和纯化(90%收率)的批处理方法。成功地将其转化为产率为90-96%的物料,并大大缩短了反应时间(20小时对1小时)并减少了化学暴露。
更新日期:2020-12-10
中文翻译:

非甾体类抗炎COX-2抑制剂塞来昔布的批次和流动合成的改进
据报道,对非甾体抗炎COX-2抑制剂塞来昔布的改进的常规分批模式合成及其流动化学选择进行了比较。塞来昔布的逐步连续流动合成是通过克莱森缩合反应获得4,4,4-三氟-1-(4-甲基-苯基)-丁烷-1,3-二酮,然后进行环缩合反应而实现的用4-磺酰胺基苯肼盐酸盐得到吡唑部分。开发了具有改进的后处理和纯化(90%收率)的批处理方法。成功地将其转化为产率为90-96%的物料,并大大缩短了反应时间(20小时对1小时)并减少了化学暴露。











































 京公网安备 11010802027423号
京公网安备 11010802027423号