当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Semiconductor nanocrystals for small molecule activation via artificial photosynthesis
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2020-11-02 , DOI: 10.1039/d0cs00930j Xu-Bing Li 1, 2, 3, 4, 5 , Zhi-Kun Xin 1, 2, 3, 4, 5 , Shu-Guang Xia 1, 2, 3, 4, 5 , Xiao-Ya Gao 1, 2, 3, 4, 5 , Chen-Ho Tung 1, 2, 3, 4, 5 , Li-Zhu Wu 1, 2, 3, 4, 5
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2020-11-02 , DOI: 10.1039/d0cs00930j Xu-Bing Li 1, 2, 3, 4, 5 , Zhi-Kun Xin 1, 2, 3, 4, 5 , Shu-Guang Xia 1, 2, 3, 4, 5 , Xiao-Ya Gao 1, 2, 3, 4, 5 , Chen-Ho Tung 1, 2, 3, 4, 5 , Li-Zhu Wu 1, 2, 3, 4, 5
Affiliation
|
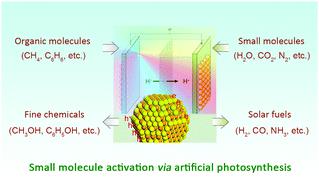
Facile activation and conversion of small molecules (e.g., H2O, CO2, N2, CH4, and C6H6) into solar fuels or value-added chemicals under mild conditions is an attractive pathway in dealing with the worldwide appeal of energy consumption and the growing demand of industrial feedstocks. Compared with conventional thermo- or electro-catalytic approaches, the protocol of photocatalysis shines light on green and low-cost storage of sunlight in chemical bonds. For instance, artificial photosynthesis is an effective way to split H2O into molecular O2 and H2, thereby storing solar energy in the form of hydrogen fuel. Because of rational tunability in band gaps, charge-carrier dynamics, exposed active sites and catalytic redox activities by tailoring size, composition, morphology, surface, and/or interface property, semiconductor nanocrystals (NCs) emerge as very promising candidates for photo-induced small molecule activation, including H2O splitting, CO2 reduction, N2 fixation, CH4 conversion and chemical bond formation (e.g., S–S, C–C, C–N, C–P, C–O). In this review, we summarize the recent advances in small molecule activation via artificial photosynthesis using semiconductor NCs, especially those consisting of II–VI and III–V elements. Moreover, we highlight the intrinsic advantages of semiconductor NCs in this field and look into the fabrication of prototype devices for large-scale and sustainable small molecule activation to store solar energy in chemical bonds.
中文翻译:

通过人工光合作用激活小分子的半导体纳米晶体
在温和条件下,小分子(例如H 2 O,CO 2,N 2,CH 4和C 6 H 6)的容易活化和转化为太阳能或高附加值化学物质是应对全球吸引力的诱人途径能源消耗和工业原料需求的增长。与传统的热催化或电催化方法相比,光催化协议将光照亮于绿色且低成本地以化学键形式存储太阳光。例如,人工光合作用是将H 2 O分解为分子O 2和H 2的有效方法,从而以氢燃料的形式存储太阳能。由于带隙,电荷载流子动力学,暴露的活性位点和催化氧化还原活性(通过调整尺寸,组成,形态,表面和/或界面性质)的合理可调性,半导体纳米晶体(NCs)成为光诱导的非常有希望的候选者小分子活化,包括H 2 O裂解,CO 2还原,N 2固定,CH 4转化和化学键形成(例如,S–S,CC–C,C–N,CP–C)。在这篇综述中,我们总结了通过小分子活化的最新进展使用半导体NC进行人工光合作用,尤其是由II–VI和III–V元素组成的NC。此外,我们重点介绍了半导体NC在该领域的固有优势,并研究了用于大规模和可持续性小分子活化以化学键存储太阳能的原型设备的制造。
更新日期:2020-11-03
中文翻译:

通过人工光合作用激活小分子的半导体纳米晶体
在温和条件下,小分子(例如H 2 O,CO 2,N 2,CH 4和C 6 H 6)的容易活化和转化为太阳能或高附加值化学物质是应对全球吸引力的诱人途径能源消耗和工业原料需求的增长。与传统的热催化或电催化方法相比,光催化协议将光照亮于绿色且低成本地以化学键形式存储太阳光。例如,人工光合作用是将H 2 O分解为分子O 2和H 2的有效方法,从而以氢燃料的形式存储太阳能。由于带隙,电荷载流子动力学,暴露的活性位点和催化氧化还原活性(通过调整尺寸,组成,形态,表面和/或界面性质)的合理可调性,半导体纳米晶体(NCs)成为光诱导的非常有希望的候选者小分子活化,包括H 2 O裂解,CO 2还原,N 2固定,CH 4转化和化学键形成(例如,S–S,CC–C,C–N,CP–C)。在这篇综述中,我们总结了通过小分子活化的最新进展使用半导体NC进行人工光合作用,尤其是由II–VI和III–V元素组成的NC。此外,我们重点介绍了半导体NC在该领域的固有优势,并研究了用于大规模和可持续性小分子活化以化学键存储太阳能的原型设备的制造。











































 京公网安备 11010802027423号
京公网安备 11010802027423号