当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unexpected Influence of Substituents on the Binding Affinities of Polycyclic Aromatic Hydrocarbons with a Tetra-Au(I) Metallorectangle
Organometallics ( IF 2.8 ) Pub Date : 2020-11-02 , DOI: 10.1021/acs.organomet.0c00639 Susana Ibáñez 1 , Dmitry G. Gusev 2 , Eduardo Peris 1
Organometallics ( IF 2.8 ) Pub Date : 2020-11-02 , DOI: 10.1021/acs.organomet.0c00639 Susana Ibáñez 1 , Dmitry G. Gusev 2 , Eduardo Peris 1
Affiliation
|
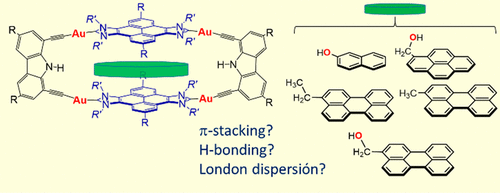
A tetragold supramolecular organometallic cage constructed with two pyrene-bis(imidazolylidene) ligands and two carbazolyl-bis(alkynyl) linkers (1) was studied as a host for a series of substituted polycyclic aromatic hydrocarbons (PAHs). For the two smaller PAHs used (2-naphthalenol and 1-pyrenemethanol), the presence of the −OH groups at the periphery of the molecules did not enhance the binding affinity of the guest, in comparison with the unsubstituted PAHs. This observation indicated no hydrogen bonding of these two guests with the NH of the carbazole linker, as well as negligible dispersive interactions of the substituents with the π system of 1. In the case of 3-perylenemethanol, the CH2OH group produced a significant increase in the binding affinity vs that of perylene. Similarly, 3-methylperylene shows an increased binding affinity in comparison to perylene. MN15-L/def2-QZVP calculations gave Gibbs reaction energies for the displacement of perylene from the host by the substituted perylenes becoming more exergonic in the order −1.6 (3-methylperylene) > −4.3 (3-ethylperylene) > −4.5 kcal/mol (3-perylenemethanol). The experimental and DFT results indicate that the peripheral dispersive interactions can make a significant contribution to the host–guest bonding energy, in addition to the conventional π–π stacking interactions. Our work highlights the importance of dispersive interactions in the contribution to the binding affinity of host–guest chemistry complexes.
中文翻译:

取代基对四环金(I)金属矩形的多环芳烃键合亲和力的意外影响
研究了由两个pyr-双(咪唑基亚基)配体和两个咔唑基-双(炔基)连接体(1)构成的四金超分子有机金属笼,作为一系列取代的多环芳烃(PAHs)的主体。对于使用的两种较小的PAH(2-萘酚和1-苯二甲醇),与未取代的PAH相比,在分子外围存在-OH基并没有增强客体的结合亲和力。该观察结果表明这两个客体与咔唑连接基的NH没有氢键键合,并且取代基与1的π系统的分散性相互作用可忽略不计。在3-per甲醇中,CH 2与per相比,OH基团的结合亲和力显着提高。类似地,与per相比,3-甲基per显示出增加的结合亲和力。MN15-L / def2-QZVP计算给出了吉布斯反应能,用于被取代的per原子以-1.6(3-甲基per)> -4.3(3-乙基per)> -4.5 kcal /摩尔(3-per甲醇)。实验和DFT结果表明,除了常规的π-π堆积相互作用之外,外围色散相互作用还可以对主客体键合能做出重要贡献。我们的工作突显了分散相互作用对宿主-客体化学复合物结合亲和力的贡献的重要性。
更新日期:2020-11-23
中文翻译:

取代基对四环金(I)金属矩形的多环芳烃键合亲和力的意外影响
研究了由两个pyr-双(咪唑基亚基)配体和两个咔唑基-双(炔基)连接体(1)构成的四金超分子有机金属笼,作为一系列取代的多环芳烃(PAHs)的主体。对于使用的两种较小的PAH(2-萘酚和1-苯二甲醇),与未取代的PAH相比,在分子外围存在-OH基并没有增强客体的结合亲和力。该观察结果表明这两个客体与咔唑连接基的NH没有氢键键合,并且取代基与1的π系统的分散性相互作用可忽略不计。在3-per甲醇中,CH 2与per相比,OH基团的结合亲和力显着提高。类似地,与per相比,3-甲基per显示出增加的结合亲和力。MN15-L / def2-QZVP计算给出了吉布斯反应能,用于被取代的per原子以-1.6(3-甲基per)> -4.3(3-乙基per)> -4.5 kcal /摩尔(3-per甲醇)。实验和DFT结果表明,除了常规的π-π堆积相互作用之外,外围色散相互作用还可以对主客体键合能做出重要贡献。我们的工作突显了分散相互作用对宿主-客体化学复合物结合亲和力的贡献的重要性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号