当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Cleavage Profile of Protein Substrates by ClpXP Reveals Deliberate Starts and Pauses
Biochemistry ( IF 2.9 ) Pub Date : 2020-11-02 , DOI: 10.1021/acs.biochem.0c00553 Catherine Y Tremblay 1 , Robert H Vass 2 , Richard W Vachet 1 , Peter Chien 2
Biochemistry ( IF 2.9 ) Pub Date : 2020-11-02 , DOI: 10.1021/acs.biochem.0c00553 Catherine Y Tremblay 1 , Robert H Vass 2 , Richard W Vachet 1 , Peter Chien 2
Affiliation
|
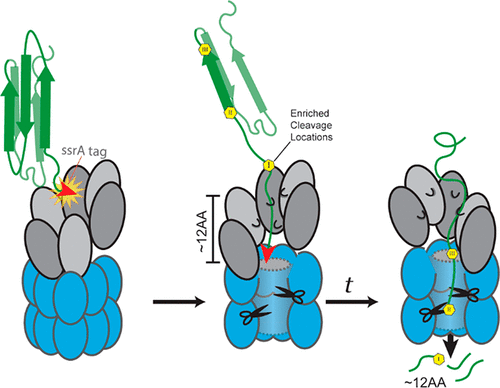
Cells rely on protein degradation by AAA+ proteases. A well-known example is the hexameric ClpX unfoldase, which captures ATP hydrolysis to feed substrates into the oligomeric ClpP peptidase. Recent studies show that an asymmetric ClpX spiral cycles protein translocation upon ATP hydrolysis. However, how this cycle affects peptide products is less explored in part because ClpP cleavage is thought to be solely defined by sequence constraints. Here, we comprehensively characterize peptides from Caulobacter crescentus ClpXP degradation of three different substrates using high-resolution mass spectrometry and find that cleavage of translocated substrates is driven by factors other than sequence. We report that defined locations in a translocated protein are especially sensitive to cleavage spaced on average every 10–13 residues. These sites are not exclusively controlled by sequence and are independent of bulk changes in catalytic peptidase sites, ATP hydrolysis, or the efficiency of initial recognition. These results fit a model in which processive translocation through ClpX starts at a specific location in a polypeptide and pauses during reset of the ClpX hexamer after a cycle of translocation. Our work suggests that defined peptides, which could be used as signaling molecules, can be generated from a given substrate by a nonspecific peptidase.
中文翻译:

ClpXP 对蛋白质底物的切割曲线揭示了故意开始和暂停
细胞依赖于 AAA+ 蛋白酶对蛋白质的降解。一个众所周知的例子是六聚体 ClpX 去折叠酶,它捕获 ATP 水解以将底物供给到寡聚体 ClpP 肽酶中。最近的研究表明,不对称的 ClpX 螺旋在 ATP 水解时循环蛋白质易位。然而,这个循环如何影响肽产物的研究较少,部分原因是 ClpP 裂解被认为仅由序列约束定义。在这里,我们全面表征了来自Caulobacter crescentus 的肽ClpXP 使用高分辨率质谱法降解三种不同的底物,发现易位底物的裂解是由序列以外的因素驱动的。我们报告说,易位蛋白质中的定义位置对平均每 10-13 个残基间隔的切割特别敏感。这些位点不仅受序列控制,而且与催化肽酶位点、ATP 水解或初始识别效率的大量变化无关。这些结果符合一个模型,其中通过 ClpX 的进行性易位从多肽中的特定位置开始,并在易位循环后 ClpX 六聚体的重置期间暂停。我们的工作表明,可用作信号分子的特定肽可以通过非特异性肽酶从给定的底物产生。
更新日期:2020-11-12
中文翻译:

ClpXP 对蛋白质底物的切割曲线揭示了故意开始和暂停
细胞依赖于 AAA+ 蛋白酶对蛋白质的降解。一个众所周知的例子是六聚体 ClpX 去折叠酶,它捕获 ATP 水解以将底物供给到寡聚体 ClpP 肽酶中。最近的研究表明,不对称的 ClpX 螺旋在 ATP 水解时循环蛋白质易位。然而,这个循环如何影响肽产物的研究较少,部分原因是 ClpP 裂解被认为仅由序列约束定义。在这里,我们全面表征了来自Caulobacter crescentus 的肽ClpXP 使用高分辨率质谱法降解三种不同的底物,发现易位底物的裂解是由序列以外的因素驱动的。我们报告说,易位蛋白质中的定义位置对平均每 10-13 个残基间隔的切割特别敏感。这些位点不仅受序列控制,而且与催化肽酶位点、ATP 水解或初始识别效率的大量变化无关。这些结果符合一个模型,其中通过 ClpX 的进行性易位从多肽中的特定位置开始,并在易位循环后 ClpX 六聚体的重置期间暂停。我们的工作表明,可用作信号分子的特定肽可以通过非特异性肽酶从给定的底物产生。











































 京公网安备 11010802027423号
京公网安备 11010802027423号