当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecularly Engineered Strong Metal Oxide–Support Interaction Enables Highly Efficient and Stable CO2 Electroreduction
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-11-01 , DOI: 10.1021/acscatal.0c03831 Lu-Pan Yuan 1, 2 , Wen-Jie Jiang 1 , Xiao-Long Liu 1 , Ye-Heng He 1 , Chao He 1, 2 , Tang Tang 1, 2 , Jianan Zhang 3 , Jin-Song Hu 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-11-01 , DOI: 10.1021/acscatal.0c03831 Lu-Pan Yuan 1, 2 , Wen-Jie Jiang 1 , Xiao-Long Liu 1 , Ye-Heng He 1 , Chao He 1, 2 , Tang Tang 1, 2 , Jianan Zhang 3 , Jin-Song Hu 1, 2
Affiliation
|
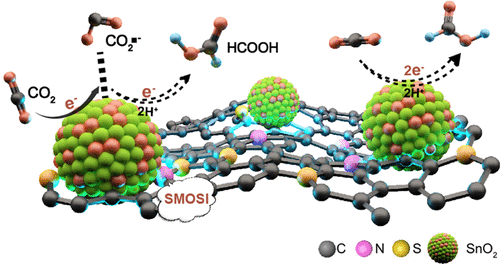
Strong metal–support interaction (SMSI), commonly happening between metal and metal oxide support, has drawn significant attention in heterogeneous catalysis due to its capability of enhancing the activity and stability of catalysts. Herein, the strong interaction between metal oxide and carbon supports is discovered to significantly boost the performance for electrocatalytic CO2 reduction reaction (CO2RR). A molecular engineering strategy is designed to develop undoped, N-doped, S-doped, and N,S-codoped porous carbon supports with similar physical properties (denoted as C, NC, SC, and NSC, respectively). These supports can host high-density SnO2 nanoparticles (over 60 wt. %) in a small size of ∼3.5 nm and good distribution, providing an excellent platform to understand the strong metal oxide–support interaction (SMOSI) and their influence on electrocatalytic performance. Systematic experimental and theoretical investigations discover the SMOSI between SnO2 nanoparticles and carbon supports in an order of SnO2/NSC > SnO2/NC > SnO2/SC > SnO2/C. Such SMOSI enables the effective electron transfer from carbon support to SnO2 nanoparticles, strengthening the adsorption of key reaction intermediate of CO2•– and thus promoting CO2RR. With the strongest SMOSI, SnO2/NSC exhibits significantly enhanced selectivity and activity for CO2 reduction to HCOOH with a high faradaic efficiency of 94.4% and an extraordinary partial current density of 56.0 mA·cm–2 in an H-cell, outperforming the majority of Sn-based catalysts. Notably, SMOSI can simultaneously secure the active sites and thus remarkably enhance their catalytic durability, making it a promising strategy for exploring efficient and stable catalysts for diverse applications.
中文翻译:

分子工程设计的强金属氧化物-支持相互作用可实现高效稳定的CO 2电还原
金属与金属氧化物载体之间通常发生的强金属-载体相互作用(SMSI)由于具有增强催化剂活性和稳定性的能力而在非均相催化中引起了极大的关注。在本文中,发现金属氧化物和碳载体之间的强相互作用显着提高了电催化CO 2还原反应(CO 2 RR)的性能。设计了分子工程学策略来开发具有相似物理特性(分别表示为C,NC,SC和NSC)的未掺杂,N掺杂,S掺杂和N,S掺杂的多孔碳载体。这些支架可以容纳高密度的SnO 2纳米颗粒(超过60 wt。%),尺寸小至约3.5 nm,分布良好,为理解强金属氧化物-载体相互作用(SMOSI)及其对电催化性能的影响提供了一个极好的平台。系统的实验和理论研究发现SnO 2纳米颗粒和碳载体之间的SMOSI顺序为SnO 2 / NSC> SnO 2 / NC> SnO 2 / SC> SnO 2 / C。这种SMOSI使电子能够有效地从碳载体转移到SnO 2纳米颗粒,从而增强了CO 2 •的关键反应中间体的吸附,从而促进了CO 2 RR的产生。拥有最强的SMOSI SnO2 / NSC在H电池中具有94.4%的高法拉第效率和56.0 mA·cm –2的非凡分流密度,显着提高了CO 2还原为HCOOH的选择性和活性,其性能优于大多数Sn基催化剂。值得注意的是,SMOSI可以同时固定活性位,从而显着提高其催化耐久性,这使其成为探索有效且稳定的催化剂以用于各种应用的有前途的策略。
更新日期:2020-11-21
中文翻译:

分子工程设计的强金属氧化物-支持相互作用可实现高效稳定的CO 2电还原
金属与金属氧化物载体之间通常发生的强金属-载体相互作用(SMSI)由于具有增强催化剂活性和稳定性的能力而在非均相催化中引起了极大的关注。在本文中,发现金属氧化物和碳载体之间的强相互作用显着提高了电催化CO 2还原反应(CO 2 RR)的性能。设计了分子工程学策略来开发具有相似物理特性(分别表示为C,NC,SC和NSC)的未掺杂,N掺杂,S掺杂和N,S掺杂的多孔碳载体。这些支架可以容纳高密度的SnO 2纳米颗粒(超过60 wt。%),尺寸小至约3.5 nm,分布良好,为理解强金属氧化物-载体相互作用(SMOSI)及其对电催化性能的影响提供了一个极好的平台。系统的实验和理论研究发现SnO 2纳米颗粒和碳载体之间的SMOSI顺序为SnO 2 / NSC> SnO 2 / NC> SnO 2 / SC> SnO 2 / C。这种SMOSI使电子能够有效地从碳载体转移到SnO 2纳米颗粒,从而增强了CO 2 •的关键反应中间体的吸附,从而促进了CO 2 RR的产生。拥有最强的SMOSI SnO2 / NSC在H电池中具有94.4%的高法拉第效率和56.0 mA·cm –2的非凡分流密度,显着提高了CO 2还原为HCOOH的选择性和活性,其性能优于大多数Sn基催化剂。值得注意的是,SMOSI可以同时固定活性位,从而显着提高其催化耐久性,这使其成为探索有效且稳定的催化剂以用于各种应用的有前途的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号