当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Understanding of the Pd(0)-Catalyzed Coupling Cyclization of 1,2-Allenyl Ketones with Aryl Halides: A Computational Study
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-10-30 , DOI: 10.1021/acscatal.0c02941 Kang Lv 1, 2 , Ping Dai 1 , Xiaoguang Bao 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-10-30 , DOI: 10.1021/acscatal.0c02941 Kang Lv 1, 2 , Ping Dai 1 , Xiaoguang Bao 1
Affiliation
|
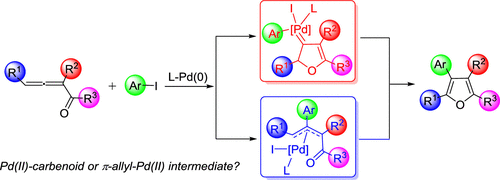
The Pd(0)-catalyzed coupling cyclization of 1,2-allenyl ketones with aryl halides to construct multisubstituted furan derivatives has been developed. Nevertheless, the detailed mechanism of this reaction, proceeding via either the Pd(II)-carbenoid or the π-allyl-Pd(II) intermediate, still remains debatable. Herein, computational studies were performed to provide mechanistic insights into the reactions, and substituent-dependent mechanistic pathways were revealed. Computational results suggest that the substituents, R1–R3 attached to 1,2-allenyl ketones and R4 attached to the aryl moiety of aryl halides, could play significant roles in the variation of the mechanistic pathway. It would be favorable to form the Pd(II)-carbenoid intermediate when R1 is an aryl/alkyl group or steric hindrance is present between R2 and R3. For the substrate of aryl halide, the aryl moiety bearing electron-donating group (R4) at the para position is more ready to undergo migratory insertion to form the π-allyl-Pd(II) intermediate than that with electron-withdrawing group. The factors responsible for the formation of both Pd(II)-carbenoid and π-allyl-Pd(II) intermediates are discussed.
中文翻译:

机械理解的Pd(0)催化1,2-烯基酮与芳基卤化物的偶联环化:计算研究。
已经开发了Pd(0)催化1,2-烯基酮与芳基卤化物的偶联环化反应,以构建多取代的呋喃衍生物。然而,该反应通过Pd(II)-类胡萝卜素或π-烯丙基-Pd(II)中间体进行的详细机理仍然值得商de。本文中,进行了计算研究以提供对反应的机理见解,并且揭示了依赖于取代基的机理。计算结果表明,取代基R 1 –R 3与1,2-烯基酮相连,R 4与芳基卤化物的芳基相连,可能在机理的变化中起重要作用。当R 1时,形成Pd(II)-类胡萝卜素中间体是有利的R 2是R 2和R 3之间的芳基/烷基或空间位阻。对于芳基卤的所述基板,所述芳基部分轴承供电子基团(R 4在)对位置更愿意接受洄游插入以形成π烯丙基钯(II)比用吸电子基团中间体。讨论了同时形成Pd(II)-类胡萝卜素和π-烯丙基-Pd(II)中间体的因素。
更新日期:2020-11-21
中文翻译:

机械理解的Pd(0)催化1,2-烯基酮与芳基卤化物的偶联环化:计算研究。
已经开发了Pd(0)催化1,2-烯基酮与芳基卤化物的偶联环化反应,以构建多取代的呋喃衍生物。然而,该反应通过Pd(II)-类胡萝卜素或π-烯丙基-Pd(II)中间体进行的详细机理仍然值得商de。本文中,进行了计算研究以提供对反应的机理见解,并且揭示了依赖于取代基的机理。计算结果表明,取代基R 1 –R 3与1,2-烯基酮相连,R 4与芳基卤化物的芳基相连,可能在机理的变化中起重要作用。当R 1时,形成Pd(II)-类胡萝卜素中间体是有利的R 2是R 2和R 3之间的芳基/烷基或空间位阻。对于芳基卤的所述基板,所述芳基部分轴承供电子基团(R 4在)对位置更愿意接受洄游插入以形成π烯丙基钯(II)比用吸电子基团中间体。讨论了同时形成Pd(II)-类胡萝卜素和π-烯丙基-Pd(II)中间体的因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号