当前位置:
X-MOL 学术
›
J. Label. Comp. Radiopharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High‐yield synthesis of a tau PET radioligand and its nonradioactive ligand using an alternative protection and deprotection strategy
Journal of Labelled Compounds and Radiopharmaceuticals ( IF 0.9 ) Pub Date : 2020-10-30 , DOI: 10.1002/jlcr.3894 Hyunjung Kim 1 , Yearn Seong Choe 1, 2
Journal of Labelled Compounds and Radiopharmaceuticals ( IF 0.9 ) Pub Date : 2020-10-30 , DOI: 10.1002/jlcr.3894 Hyunjung Kim 1 , Yearn Seong Choe 1, 2
Affiliation
|
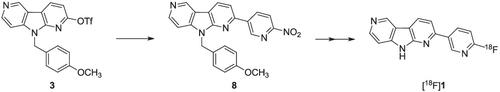
Recently developed tau imaging radiopharmaceuticals show specific uptake in tau protein‐rich regions in human brains without off‐target binding. These radiopharmaceuticals and their nonradioactive reference ligands are generally obtained in low (radio)chemical yields. In the present study, we investigated high‐yield synthesis of 18F‐RO948 ([18F]1) and its nonradioactive ligand (1). The ligand 1 was synthesized by a Suzuki‐Miyaura coupling reaction between 9‐(4‐methoxybenzyl)‐9H‐pyrrolo[2,3‐b:4,5‐c′]dipyridin‐2‐yl trifluoromethanesulfonate (3) and 2‐fluoro‐5‐(4,4,5,5‐tetramethyl‐1,3,2‐dioxaborolan‐2‐yl)pyridine (4), followed by oxidative removal of the para‐methoxybenzyl (PMB) group with ceric ammonium nitrate (CAN). This two‐step reaction gave 1 in 55.8% yield. The precursor for [18F]1 was synthesized from 3 and 2‐nitro‐5‐(4,4,5,5‐tetramethyl‐1,3,2‐dioxaborolan‐2‐yl)pyridine (6). The resulting PMB‐protected precursor 8 was obtained in 74.5% yield. [18F]1 was synthesized by radiofluorination of 8 (radiochemical conversion (RCC): 95.7 ± 1.7%), followed by deprotection of the PMB group with CAN. This one‐pot, two‐step radiochemical synthesis followed by HPLC purification gave [18F]1 in high decay‐corrected radiochemical yield (54‐60%). The RCC of [18F]fluoride to [18F]1 in our two‐step synthesis method was similar to that in a one‐step radiofluorination reaction of a tert‐butoxycarbonyl (BOC)‐protected precursor 10 that proceeds with concomitant thermal deprotection of the BOC group. Taken together, the results of this study suggest that this high‐yield synthesis method is useful for the synthesis of 18F‐labeled (NH)heteroarene compounds.
中文翻译:

使用替代保护和去保护策略高产率合成 tau PET 放射性配体及其非放射性配体
最近开发的 tau 成像放射性药物在人脑中富含 tau 蛋白的区域显示出特异性摄取,而没有脱靶结合。这些放射性药物及其非放射性参考配体通常以低(放射性)化学产率获得。在本研究中,我们研究了18 F-RO948 ([ 18 F] 1 ) 及其非放射性配体 ( 1 )的高产合成。配体1通过 9-(4-methoxybenzyl)-9 H -pyrrolo[2,3-b:4,5-c']dipyridin-2-yl trifluoromethanesulfonate ( 3 ) 和 2之间的 Suzuki-Miyaura 偶联反应合成-氟-5-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)吡啶( 4),然后用硝酸铈铵 (CAN) 氧化去除对甲氧基苄基 (PMB) 基团。这个两步反应以 55.8% 的收率得到1。[ 18 F] 1的前体由3和 2-硝基-5-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)吡啶 ( 6 ) 合成。得到的 PMB 保护的前体8的产率为 74.5%。[ 18 F] 1是通过8的放射性氟化(放射化学转化率 (RCC):95.7 ± 1.7%)合成的,然后用 CAN 对 PMB 基团进行去保护。这种一锅法、两步放射化学合成,然后是 HPLC 纯化得到 [18 F] 1具有高衰减校正放射化学产率 (54-60%)。在我们的两步合成方法中,[ 18 F] 氟化物到 [ 18 F] 1的 RCC类似于叔丁氧羰基 (BOC) 保护的前体10的一步放射性氟化反应,同时伴随着热脱保护中银集团。综上所述,本研究结果表明,这种高产率合成方法可用于合成18 F 标记的 (NH) 杂芳烃化合物。
更新日期:2020-10-30
中文翻译:

使用替代保护和去保护策略高产率合成 tau PET 放射性配体及其非放射性配体
最近开发的 tau 成像放射性药物在人脑中富含 tau 蛋白的区域显示出特异性摄取,而没有脱靶结合。这些放射性药物及其非放射性参考配体通常以低(放射性)化学产率获得。在本研究中,我们研究了18 F-RO948 ([ 18 F] 1 ) 及其非放射性配体 ( 1 )的高产合成。配体1通过 9-(4-methoxybenzyl)-9 H -pyrrolo[2,3-b:4,5-c']dipyridin-2-yl trifluoromethanesulfonate ( 3 ) 和 2之间的 Suzuki-Miyaura 偶联反应合成-氟-5-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)吡啶( 4),然后用硝酸铈铵 (CAN) 氧化去除对甲氧基苄基 (PMB) 基团。这个两步反应以 55.8% 的收率得到1。[ 18 F] 1的前体由3和 2-硝基-5-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)吡啶 ( 6 ) 合成。得到的 PMB 保护的前体8的产率为 74.5%。[ 18 F] 1是通过8的放射性氟化(放射化学转化率 (RCC):95.7 ± 1.7%)合成的,然后用 CAN 对 PMB 基团进行去保护。这种一锅法、两步放射化学合成,然后是 HPLC 纯化得到 [18 F] 1具有高衰减校正放射化学产率 (54-60%)。在我们的两步合成方法中,[ 18 F] 氟化物到 [ 18 F] 1的 RCC类似于叔丁氧羰基 (BOC) 保护的前体10的一步放射性氟化反应,同时伴随着热脱保护中银集团。综上所述,本研究结果表明,这种高产率合成方法可用于合成18 F 标记的 (NH) 杂芳烃化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号