当前位置:
X-MOL 学术
›
Aging Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regulatory feedback cycle of the insulin‐degrading enzyme and the amyloid precursor protein intracellular domain: Implications for Alzheimer’s disease
Aging Cell ( IF 8.0 ) Pub Date : 2020-10-31 , DOI: 10.1111/acel.13264 Anna A Lauer 1 , Janine Mett 1, 2 , Daniel Janitschke 1 , Andrea Thiel 1 , Christoph P Stahlmann 1 , Cornel M Bachmann 1 , Felix Ritzmann 3 , Bianca Schrul 4 , Ulrike C Müller 5 , Reuven Stein 6 , Matthias Riemenschneider 7 , Heike S Grimm 1 , Tobias Hartmann 1, 8 , Marcus O W Grimm 1, 8
Aging Cell ( IF 8.0 ) Pub Date : 2020-10-31 , DOI: 10.1111/acel.13264 Anna A Lauer 1 , Janine Mett 1, 2 , Daniel Janitschke 1 , Andrea Thiel 1 , Christoph P Stahlmann 1 , Cornel M Bachmann 1 , Felix Ritzmann 3 , Bianca Schrul 4 , Ulrike C Müller 5 , Reuven Stein 6 , Matthias Riemenschneider 7 , Heike S Grimm 1 , Tobias Hartmann 1, 8 , Marcus O W Grimm 1, 8
Affiliation
|
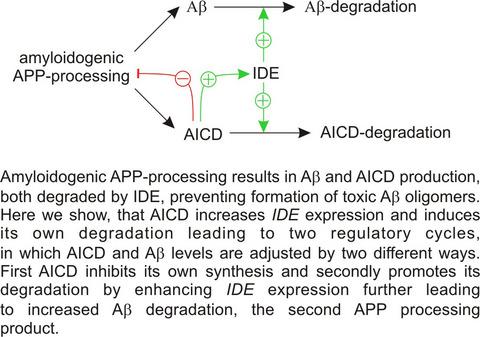
One of the major pathological hallmarks of Alzheimer´s disease (AD) is an accumulation of amyloid‐β (Aβ) in brain tissue leading to formation of toxic oligomers and senile plaques. Under physiological conditions, a tightly balanced equilibrium between Aβ‐production and ‐degradation is necessary to prevent pathological Aβ‐accumulation. Here, we investigate the molecular mechanism how insulin‐degrading enzyme (IDE), one of the major Aβ‐degrading enzymes, is regulated and how amyloid precursor protein (APP) processing and Aβ‐degradation is linked in a regulatory cycle to achieve this balance. In absence of Aβ‐production caused by APP or Presenilin deficiency, IDE‐mediated Aβ‐degradation was decreased, accompanied by a decreased IDE activity, protein level, and expression. Similar results were obtained in cells only expressing a truncated APP, lacking the APP intracellular domain (AICD) suggesting that AICD promotes IDE expression. In return, APP overexpression mediated an increased IDE expression, comparable results were obtained with cells overexpressing C50, a truncated APP representing AICD. Beside these genetic approaches, also AICD peptide incubation and pharmacological inhibition of the γ‐secretase preventing AICD production regulated IDE expression and promoter activity. By utilizing CRISPR/Cas9 APP and Presenilin knockout SH‐SY5Y cells results were confirmed in a second cell line in addition to mouse embryonic fibroblasts. In vivo, IDE expression was decreased in mouse brains devoid of APP or AICD, which was in line with a significant correlation of APP expression level and IDE expression in human postmortem AD brains. Our results show a tight link between Aβ‐production and Aβ‐degradation forming a regulatory cycle in which AICD promotes Aβ‐degradation via IDE and IDE itself limits its own production by degrading AICD.
中文翻译:

胰岛素降解酶和淀粉样前体蛋白胞内结构域的调节反馈循环:对阿尔茨海默病的影响
阿尔茨海默病 (AD) 的主要病理特征之一是脑组织中淀粉样蛋白 β (Aβ) 的积累,导致有毒低聚物和老年斑的形成。在生理条件下,Aβ 产生和降解之间的紧密平衡是防止病理性 Aβ 积累所必需的。在这里,我们研究了胰岛素降解酶 (IDE)(主要的 Aβ 降解酶之一)如何受到调节的分子机制,以及淀粉样前体蛋白 (APP) 加工和 Aβ 降解如何在调节循环中联系起来以实现这种平衡. 在缺乏由 APP 或早老素缺乏引起的 Aβ 产生的情况下,IDE 介导的 Aβ 降解减少,同时伴随着 IDE 活性、蛋白质水平和表达的降低。在仅表达截短的 APP 的细胞中获得了类似的结果,IDE表达式。作为回报,APP过表达介导了IDE表达的增加,与过表达 C50(一种代表 AICD 的截短 APP)的细胞获得了类似的结果。除了这些遗传方法之外,AICD 肽孵育和 γ-分泌酶的药理学抑制也可以调节IDE表达和启动子活性。通过利用 CRISPR/Cas9 APP 和 Presenilin 敲除 SH-SY5Y 细胞,结果在除小鼠胚胎成纤维细胞之外的第二个细胞系中得到证实。在体内,缺乏APP或AICD的小鼠大脑中IDE表达降低,这与APP表达水平与IDE的显着相关性一致在人类死后AD 大脑中的表达。我们的结果显示 Aβ 产生和 Aβ 降解之间存在紧密联系,形成一个调节循环,其中 AICD 通过 IDE 促进 Aβ 降解,IDE 本身通过降解 AICD 来限制其自身的产生。
更新日期:2020-11-23
中文翻译:

胰岛素降解酶和淀粉样前体蛋白胞内结构域的调节反馈循环:对阿尔茨海默病的影响
阿尔茨海默病 (AD) 的主要病理特征之一是脑组织中淀粉样蛋白 β (Aβ) 的积累,导致有毒低聚物和老年斑的形成。在生理条件下,Aβ 产生和降解之间的紧密平衡是防止病理性 Aβ 积累所必需的。在这里,我们研究了胰岛素降解酶 (IDE)(主要的 Aβ 降解酶之一)如何受到调节的分子机制,以及淀粉样前体蛋白 (APP) 加工和 Aβ 降解如何在调节循环中联系起来以实现这种平衡. 在缺乏由 APP 或早老素缺乏引起的 Aβ 产生的情况下,IDE 介导的 Aβ 降解减少,同时伴随着 IDE 活性、蛋白质水平和表达的降低。在仅表达截短的 APP 的细胞中获得了类似的结果,IDE表达式。作为回报,APP过表达介导了IDE表达的增加,与过表达 C50(一种代表 AICD 的截短 APP)的细胞获得了类似的结果。除了这些遗传方法之外,AICD 肽孵育和 γ-分泌酶的药理学抑制也可以调节IDE表达和启动子活性。通过利用 CRISPR/Cas9 APP 和 Presenilin 敲除 SH-SY5Y 细胞,结果在除小鼠胚胎成纤维细胞之外的第二个细胞系中得到证实。在体内,缺乏APP或AICD的小鼠大脑中IDE表达降低,这与APP表达水平与IDE的显着相关性一致在人类死后AD 大脑中的表达。我们的结果显示 Aβ 产生和 Aβ 降解之间存在紧密联系,形成一个调节循环,其中 AICD 通过 IDE 促进 Aβ 降解,IDE 本身通过降解 AICD 来限制其自身的产生。











































 京公网安备 11010802027423号
京公网安备 11010802027423号