Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A high‐throughput genetically directed protein crosslinking analysis reveals the physiological relevance of the ATP synthase ‘inserted’ state
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-10-31 , DOI: 10.1111/febs.15616 Yang Liu 1 , Jiayu Yu 1 , Mengyuan Wang 1 , Qingfang Zeng 1 , Xinmiao Fu 1 , Zengyi Chang 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-10-31 , DOI: 10.1111/febs.15616 Yang Liu 1 , Jiayu Yu 1 , Mengyuan Wang 1 , Qingfang Zeng 1 , Xinmiao Fu 1 , Zengyi Chang 1
Affiliation
|
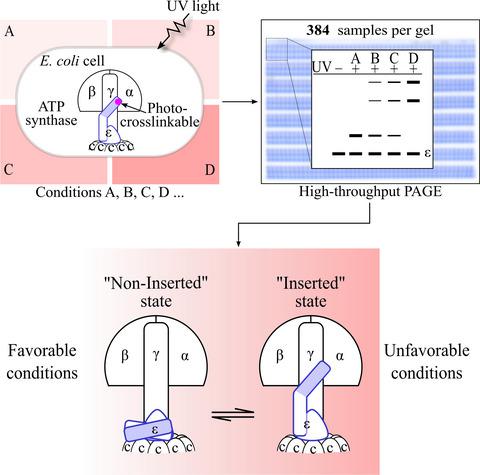
ATP synthase, a highly conserved protein complex that has a subunit composition of α3β3γδεab2c8–15 for the bacterial enzyme, is a key player in supplying energy to living organisms. This protein complex consists of a peripheral F1 sector (α3β3γδε) and a membrane‐integrated Fo sector (ab2c8–15). Structural analyses of the isolated protein components revealed that, remarkably, the C‐terminal domain of its ε‐subunit seems to adopt two dramatically different structures, but the physiological relevance of this conformational change remains largely unknown. In an attempt to decipher this, we developed a high‐throughput in vivo protein photo‐cross‐linking analysis pipeline based on the introduction of the unnatural amino acid into the target protein via the scarless genome‐targeted site‐directed mutagenesis technique, and probing the cross‐linked products via the high‐throughput polyacrylamide gel electrophoresis technique. Employing this pipeline, we examined the interactions involving the C‐terminal helix of the ε‐subunit in cells living under a variety of experimental conditions. These studies enabled us to uncover that the bacterial ATP synthase exists as an equilibrium between the ‘inserted’ and ‘noninserted’ state in cells, maintaining a moderate but significant level of net ATP synthesis when shifting to the former upon exposing to unfavorable energetically stressful conditions. Such a mechanism allows the bacterial ATP synthases to proportionally and instantly switch between two reversible functional states in responding to changing environmental conditions. Importantly, this high‐throughput approach could allow us to decipher the physiological relevance of protein–protein interactions identified under in vitro conditions or to unveil novel physiological context‐dependent protein–protein interactions that are unknown before.
中文翻译:

高通量遗传定向蛋白质交联分析揭示了 ATP 合酶“插入”状态的生理相关性
ATP 合酶是一种高度保守的蛋白质复合物,其细菌酶的亚基组成为 α 3 β 3 γδεab 2 c 8–15 ,是为生物体提供能量的关键角色。该蛋白质复合物由外周 F 1扇区 (α 3 β 3 γδε) 和膜整合的 F o扇区 (ab 2 c 8-15 ) 组成。对分离的蛋白质成分的结构分析表明,值得注意的是,其 ε 亚基的 C 末端结构域似乎采用了两种截然不同的结构,但这种构象变化的生理相关性仍然很大程度上未知。为了解释这一点,我们开发了一种高通量体内蛋白质光交联分析管道,其基础是通过无痕基因组靶向定点诱变技术将非天然氨基酸引入到目标蛋白质中,并探测通过高通量聚丙烯酰胺凝胶电泳技术得到交联产物。利用该流程,我们检查了在各种实验条件下生活的细胞中涉及 ε 亚基 C 末端螺旋的相互作用。这些研究使我们发现,细菌 ATP 合酶在细胞中以“插入”和“非插入”状态之间的平衡存在,在暴露于不利的能量应激条件下转向前者时,保持适度但显着的净 ATP 合成水平。这种机制允许细菌 ATP 合酶在两种可逆功能状态之间按比例即时切换,以响应不断变化的环境条件。 重要的是,这种高通量方法可以让我们破译在体外条件下确定的蛋白质-蛋白质相互作用的生理相关性,或者揭示以前未知的新型生理背景依赖性蛋白质-蛋白质相互作用。
更新日期:2020-10-31
中文翻译:

高通量遗传定向蛋白质交联分析揭示了 ATP 合酶“插入”状态的生理相关性
ATP 合酶是一种高度保守的蛋白质复合物,其细菌酶的亚基组成为 α 3 β 3 γδεab 2 c 8–15 ,是为生物体提供能量的关键角色。该蛋白质复合物由外周 F 1扇区 (α 3 β 3 γδε) 和膜整合的 F o扇区 (ab 2 c 8-15 ) 组成。对分离的蛋白质成分的结构分析表明,值得注意的是,其 ε 亚基的 C 末端结构域似乎采用了两种截然不同的结构,但这种构象变化的生理相关性仍然很大程度上未知。为了解释这一点,我们开发了一种高通量体内蛋白质光交联分析管道,其基础是通过无痕基因组靶向定点诱变技术将非天然氨基酸引入到目标蛋白质中,并探测通过高通量聚丙烯酰胺凝胶电泳技术得到交联产物。利用该流程,我们检查了在各种实验条件下生活的细胞中涉及 ε 亚基 C 末端螺旋的相互作用。这些研究使我们发现,细菌 ATP 合酶在细胞中以“插入”和“非插入”状态之间的平衡存在,在暴露于不利的能量应激条件下转向前者时,保持适度但显着的净 ATP 合成水平。这种机制允许细菌 ATP 合酶在两种可逆功能状态之间按比例即时切换,以响应不断变化的环境条件。 重要的是,这种高通量方法可以让我们破译在体外条件下确定的蛋白质-蛋白质相互作用的生理相关性,或者揭示以前未知的新型生理背景依赖性蛋白质-蛋白质相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号