当前位置:
X-MOL 学术
›
Acta Cryst. F
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alternative conformation of the C‐domain of the P140 protein from Mycoplasma genitalium
Acta Crystallographica Section F ( IF 1.1 ) Pub Date : 2020-11-02 , DOI: 10.1107/s2053230x20012297 David Vizarraga 1 , Rosa Pérez-Luque 1 , Jesús Martín 1 , Ignacio Fita 1 , David Aparicio 1
Acta Crystallographica Section F ( IF 1.1 ) Pub Date : 2020-11-02 , DOI: 10.1107/s2053230x20012297 David Vizarraga 1 , Rosa Pérez-Luque 1 , Jesús Martín 1 , Ignacio Fita 1 , David Aparicio 1
Affiliation
|
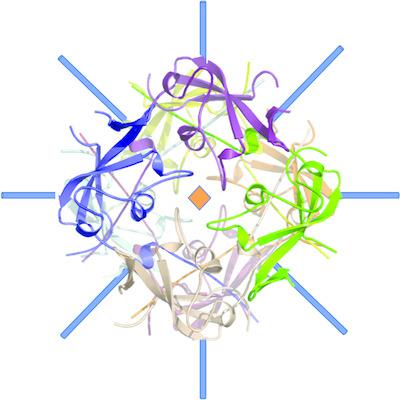
The human pathogen Mycoplasma genitalium is responsible for urethritis in men, and for cervicitis and pelvic inflammatory disease in women. The adherence of M. genitalium to host target epithelial cells is mediated through an adhesion complex called Nap, which is essential for infectivity. Nap is a transmembrane dimer of heterodimers of the immunodominant proteins P110 and P140. The M. genitalium genome contains multiple copies of portions that share homology with the extracellular regions of P140 and P110 encoded by the genes mg191 and mg192, respectively. Homologous recombination between the genes and the copies allows the generation of a large diversity of P140 and P110 variants to overcome surveillance by the host immune system. Interestingly, the C‐terminal domain (C‐domain) of the extracellular region of P140, which is essential for the function of Nap by acting as a flexible stalk anchoring the protein to the mycoplasma membrane, presents a low degree of sequence variability. In the present work, the X‐ray crystal structures of two crystal forms of a construct of the P140 C‐domain are reported. In both crystal forms, the construct forms a compact octamer with D4 point‐group symmetry. The structure of the C‐domain determined in this work presents significant differences with respect to the structure of the C‐domain found recently in intact P140. The structural plasticity of the C‐domain appears to be a possible mechanism that may help in the functioning of the mycoplasma adhesion complex.
中文翻译:

生殖支原体 P140 蛋白 C 结构域的替代构象
人类病原体生殖支原体导致男性尿道炎、女性宫颈炎和盆腔炎。生殖支原体与宿主靶上皮细胞的粘附是通过一种称为 Nap 的粘附复合物介导的,这对于感染性至关重要。 Nap 是免疫显性蛋白 P110 和 P140 异二聚体的跨膜二聚体。生殖支原体基因组包含与分别由基因mg191和mg192编码的 P140 和 P110 胞外区域具有同源性的部分的多个拷贝。基因和拷贝之间的同源重组允许产生大量多样性的 P140 和 P110 变体,以克服宿主免疫系统的监视。有趣的是,P140 胞外区的 C 端结构域(C 结构域)具有较低程度的序列变异性,该结构域作为将蛋白质锚定到支原体膜上的柔性茎,对于 Nap 的功能至关重要。在本工作中,报道了 P140 C 结构域结构的两种晶型的 X 射线晶体结构。在这两种晶体形式中,该结构形成具有D 4 点群对称性的紧凑八聚体。本工作中确定的 C 结构域结构与最近在完整 P140 中发现的 C 结构域结构存在显着差异。 C 结构域的结构可塑性似乎是一种可能有助于支原体粘附复合物发挥功能的机制。
更新日期:2020-11-02
中文翻译:

生殖支原体 P140 蛋白 C 结构域的替代构象
人类病原体生殖支原体导致男性尿道炎、女性宫颈炎和盆腔炎。生殖支原体与宿主靶上皮细胞的粘附是通过一种称为 Nap 的粘附复合物介导的,这对于感染性至关重要。 Nap 是免疫显性蛋白 P110 和 P140 异二聚体的跨膜二聚体。生殖支原体基因组包含与分别由基因mg191和mg192编码的 P140 和 P110 胞外区域具有同源性的部分的多个拷贝。基因和拷贝之间的同源重组允许产生大量多样性的 P140 和 P110 变体,以克服宿主免疫系统的监视。有趣的是,P140 胞外区的 C 端结构域(C 结构域)具有较低程度的序列变异性,该结构域作为将蛋白质锚定到支原体膜上的柔性茎,对于 Nap 的功能至关重要。在本工作中,报道了 P140 C 结构域结构的两种晶型的 X 射线晶体结构。在这两种晶体形式中,该结构形成具有D 4 点群对称性的紧凑八聚体。本工作中确定的 C 结构域结构与最近在完整 P140 中发现的 C 结构域结构存在显着差异。 C 结构域的结构可塑性似乎是一种可能有助于支原体粘附复合物发挥功能的机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号