当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Neutron diffraction experiment with the Y116S variant of transthyretin using iBIX at J‐PARC: application of a new integration method
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-11-02 , DOI: 10.1107/s2059798320012498 Katsuhiro Kusaka 1 , Takeshi Yokoyama 2 , Taro Yamada 1 , Naomine Yano 1 , Ichiro Tanaka 1 , Mineyuki Mizuguchi 2
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-11-02 , DOI: 10.1107/s2059798320012498 Katsuhiro Kusaka 1 , Takeshi Yokoyama 2 , Taro Yamada 1 , Naomine Yano 1 , Ichiro Tanaka 1 , Mineyuki Mizuguchi 2
Affiliation
|
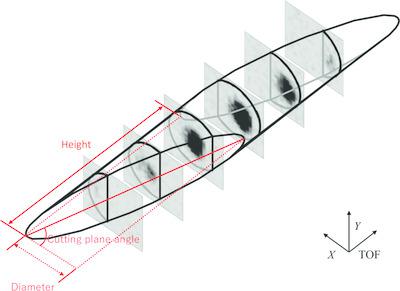
Transthyretin (TTR) is one of more than 30 amyloidogenic proteins, and the amyloid fibrils found in patients afflicted with ATTR amyloidosis are composed of this protein. Wild‐type TTR amyloids accumulate in the heart in senile systemic amyloidosis (SSA). ATTR amyloidosis occurs at a much younger age than SSA, and the affected individuals carry a TTR mutant. The naturally occurring amyloidogenic Y116S TTR variant forms more amyloid fibrils than wild‐type TTR. Thus, the Y116S mutation reduces the stability of the TTR structure. A neutron diffraction experiment on Y116S TTR was performed to elucidate the mechanism of the changes in structural stability between Y116S variant and wild‐type TTR through structural comparison. Large crystals of the Y116S variant were grown under optimal crystallization conditions, and a single 2.4 mm3 crystal was ultimately obtained. This crystal was subjected to time‐of‐flight (TOF) neutron diffraction using the IBARAKI biological crystal diffractometer (iBIX) at the Japan Proton Accelerator Research Complex, Tokai, Japan (J‐PARC). A full data set for neutron structure analysis was obtained in 14 days at an operational accelerator power of 500 kW. A new integration method was developed and showed improved data statistics; the new method was applied to the reduction of the TOF diffraction data from the Y116S variant. Data reduction was completed and the integrated intensities of the Bragg reflections were obtained at 1.9 Å resolution for structure refinement. Moreover, X‐ray diffraction data at 1.4 Å resolution were obtained for joint neutron–X‐ray refinement.
中文翻译:

在J‐PARC使用iBIX对运甲状腺素蛋白Y116S变体进行中子衍射实验:一种新的积分方法的应用
运甲状腺素蛋白(TTR)是30多种淀粉样蛋白生成蛋白之一,在ATTR淀粉样变性病患者中发现的淀粉样原纤维是由这种蛋白组成的。野生型TTR淀粉样蛋白会在老年性系统性淀粉样变性病(SSA)中积聚在心脏中。ATTR淀粉样变性病的发生年龄比SSA小得多,受影响的个体携带TTR突变体。天然产生淀粉样的Y116S TTR变异体比野生型TTR形成更多的淀粉样原纤维。因此,Y116S突变降低了TTR结构的稳定性。进行了Y116S TTR的中子衍射实验,以通过结构比较阐明Y116S变体和野生型TTR之间的结构稳定性变化的机理。Y116S变体的大晶体在最佳结晶条件下生长,并且单个2.4 mm最终获得3个晶体。使用日本Tokai日本质子加速器研究中心(J-PARC)的IBARAKI生物晶体衍射仪(iBIX)对该晶体进行飞行时间(TOF)中子衍射。在14天内以500 kW的运行加速器功率获得了用于中子结构分析的完整数据集。开发了一种新的集成方法,并显示了改进的数据统计;新方法被应用于减少来自Y116S变体的TOF衍射数据。完成了数据还原,并以1.9Å的分辨率获得了布拉格反射的积分强度,以进行结构优化。而且,获得了1.4Å分辨率的X射线衍射数据,用于联合中子– X射线精修。
更新日期:2020-11-02
中文翻译:

在J‐PARC使用iBIX对运甲状腺素蛋白Y116S变体进行中子衍射实验:一种新的积分方法的应用
运甲状腺素蛋白(TTR)是30多种淀粉样蛋白生成蛋白之一,在ATTR淀粉样变性病患者中发现的淀粉样原纤维是由这种蛋白组成的。野生型TTR淀粉样蛋白会在老年性系统性淀粉样变性病(SSA)中积聚在心脏中。ATTR淀粉样变性病的发生年龄比SSA小得多,受影响的个体携带TTR突变体。天然产生淀粉样的Y116S TTR变异体比野生型TTR形成更多的淀粉样原纤维。因此,Y116S突变降低了TTR结构的稳定性。进行了Y116S TTR的中子衍射实验,以通过结构比较阐明Y116S变体和野生型TTR之间的结构稳定性变化的机理。Y116S变体的大晶体在最佳结晶条件下生长,并且单个2.4 mm最终获得3个晶体。使用日本Tokai日本质子加速器研究中心(J-PARC)的IBARAKI生物晶体衍射仪(iBIX)对该晶体进行飞行时间(TOF)中子衍射。在14天内以500 kW的运行加速器功率获得了用于中子结构分析的完整数据集。开发了一种新的集成方法,并显示了改进的数据统计;新方法被应用于减少来自Y116S变体的TOF衍射数据。完成了数据还原,并以1.9Å的分辨率获得了布拉格反射的积分强度,以进行结构优化。而且,获得了1.4Å分辨率的X射线衍射数据,用于联合中子– X射线精修。











































 京公网安备 11010802027423号
京公网安备 11010802027423号