当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal structures of peanut lectin in the presence of synthetic β‐N‐ and β‐S‐galactosides disclose evidence for the recognition of different glycomimetic ligands
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-11-02 , DOI: 10.1107/s2059798320012371 Alejandro J Cagnoni 1 , Emiliano D Primo 2 , Sebastián Klinke 3 , María E Cano 4 , Walter Giordano 2 , Karina V Mariño 1 , José Kovensky 5 , Fernando A Goldbaum 3 , María Laura Uhrig 4 , Lisandro H Otero 3
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-11-02 , DOI: 10.1107/s2059798320012371 Alejandro J Cagnoni 1 , Emiliano D Primo 2 , Sebastián Klinke 3 , María E Cano 4 , Walter Giordano 2 , Karina V Mariño 1 , José Kovensky 5 , Fernando A Goldbaum 3 , María Laura Uhrig 4 , Lisandro H Otero 3
Affiliation
|
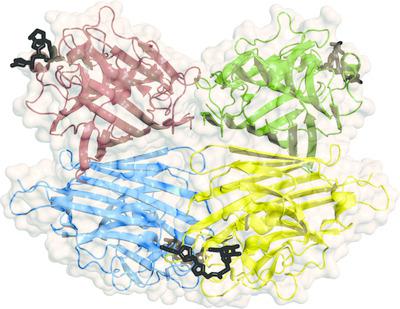
Carbohydrate–lectin interactions are involved in important cellular recognition processes, including viral and bacterial infections, inflammation and tumor metastasis. Hence, structural studies of lectin–synthetic glycan complexes are essential for understanding lectin‐recognition processes and for the further design of promising chemotherapeutics that interfere with sugar–lectin interactions. Plant lectins are excellent models for the study of the molecular‐recognition process. Among them, peanut lectin (PNA) is highly relevant in the field of glycobiology because of its specificity for β‐galactosides, showing high affinity towards the Thomsen–Friedenreich antigen, a well known tumor‐associated carbohydrate antigen. Given this specificity, PNA is one of the most frequently used molecular probes for the recognition of tumor cell‐surface O‐glycans. Thus, it has been extensively used in glycobiology for inhibition studies with a variety of β‐galactoside and β‐lactoside ligands. Here, crystal structures of PNA are reported in complex with six novel synthetic hydrolytically stable β‐N‐ and β‐S‐galactosides. These complexes disclosed key molecular‐binding interactions of the different sugars with PNA at the atomic level, revealing the roles of specific water molecules in protein–ligand recognition. Furthermore, binding‐affinity studies by isothermal titration calorimetry showed dissociation‐constant values in the micromolar range, as well as a positive multivalency effect in terms of affinity in the case of the divalent compounds. Taken together, this work provides a qualitative structural rationale for the upcoming synthesis of optimized glycoclusters designed for the study of lectin‐mediated biological processes. The understanding of the recognition of β‐N‐ and β‐S‐galactosides by PNA represents a benchmark in protein–carbohydrate interactions since they are novel synthetic ligands that do not belong to the family of O‐linked glycosides.
中文翻译:

存在合成的β-N-和β-S-半乳糖苷的花生凝集素的晶体结构揭示了识别不同的拟糖配体的证据
碳水化合物-凝集素相互作用涉及重要的细胞识别过程,包括病毒和细菌感染,炎症和肿瘤转移。因此,对凝集素合成聚糖复合物的结构研究对于理解凝集素识别过程以及进一步设计有前景的干扰糖-凝集素相互作用的化学治疗药物至关重要。植物凝集素是研究分子识别过程的优秀模型。其中,花生凝集素(PNA)因其对β-半乳糖苷的特异性而在糖生物学领域具有高度相关性,显示出对Thomsen-Friedenreich抗原(一种与肿瘤相关的碳水化合物抗原)的高亲和力。鉴于这种特异性,PNA是识别肿瘤细胞表面O-聚糖的最常用分子探针之一。因此,它已在糖生物学中广泛用于各种β-半乳糖苷和β-乳糖苷配体的抑制研究。在此,报道了PNA的晶体结构与六种新型合成的水解稳定的β-N-和β-S-半乳糖苷的复合物。这些复合物在原子水平上揭示了不同糖与PNA的关键分子结合相互作用,揭示了特定水分子在蛋白质-配体识别中的作用。此外,通过等温滴定量热法进行的结合亲和力研究表明,在微摩尔范围内的解离常数值不变,对于二价化合物,在亲和力方面具有积极的多价效应。在一起 这项工作为即将进行的用于研究凝集素介导的生物过程的优化糖簇的合成提供了定性的结构原理。由于PNA是不属于O-连接糖苷家族的新型合成配体,因此对PNA识别β-N-和β-S-半乳糖苷的理解代表了蛋白质与碳水化合物相互作用的基准。
更新日期:2020-11-02
中文翻译:

存在合成的β-N-和β-S-半乳糖苷的花生凝集素的晶体结构揭示了识别不同的拟糖配体的证据
碳水化合物-凝集素相互作用涉及重要的细胞识别过程,包括病毒和细菌感染,炎症和肿瘤转移。因此,对凝集素合成聚糖复合物的结构研究对于理解凝集素识别过程以及进一步设计有前景的干扰糖-凝集素相互作用的化学治疗药物至关重要。植物凝集素是研究分子识别过程的优秀模型。其中,花生凝集素(PNA)因其对β-半乳糖苷的特异性而在糖生物学领域具有高度相关性,显示出对Thomsen-Friedenreich抗原(一种与肿瘤相关的碳水化合物抗原)的高亲和力。鉴于这种特异性,PNA是识别肿瘤细胞表面O-聚糖的最常用分子探针之一。因此,它已在糖生物学中广泛用于各种β-半乳糖苷和β-乳糖苷配体的抑制研究。在此,报道了PNA的晶体结构与六种新型合成的水解稳定的β-N-和β-S-半乳糖苷的复合物。这些复合物在原子水平上揭示了不同糖与PNA的关键分子结合相互作用,揭示了特定水分子在蛋白质-配体识别中的作用。此外,通过等温滴定量热法进行的结合亲和力研究表明,在微摩尔范围内的解离常数值不变,对于二价化合物,在亲和力方面具有积极的多价效应。在一起 这项工作为即将进行的用于研究凝集素介导的生物过程的优化糖簇的合成提供了定性的结构原理。由于PNA是不属于O-连接糖苷家族的新型合成配体,因此对PNA识别β-N-和β-S-半乳糖苷的理解代表了蛋白质与碳水化合物相互作用的基准。











































 京公网安备 11010802027423号
京公网安备 11010802027423号