Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mucin‐Inspired, High Molecular Weight Virus Binding Inhibitors Show Biphasic Binding Behavior to Influenza A Viruses
Small ( IF 13.0 ) Pub Date : 2020-11-01 , DOI: 10.1002/smll.202004635 Matthias Wallert 1 , Chuanxiong Nie 2 , Parambath Anilkumar 3 , Srinivas Abbina 3 , Sumati Bhatia 2 , Kai Ludwig 4 , Jayachandran N. Kizhakkedathu 3, 5, 6 , Rainer Haag 2 , Stephan Block 1
Small ( IF 13.0 ) Pub Date : 2020-11-01 , DOI: 10.1002/smll.202004635 Matthias Wallert 1 , Chuanxiong Nie 2 , Parambath Anilkumar 3 , Srinivas Abbina 3 , Sumati Bhatia 2 , Kai Ludwig 4 , Jayachandran N. Kizhakkedathu 3, 5, 6 , Rainer Haag 2 , Stephan Block 1
Affiliation
|
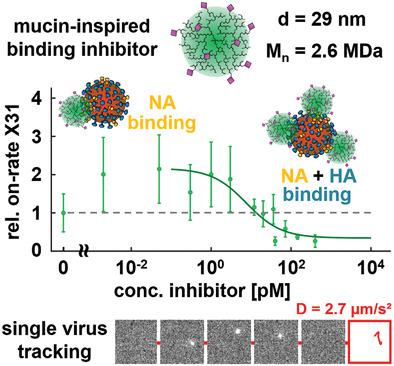
Multivalent binding inhibitors are a promising new class of antivirals that prevent virus infections by inhibiting virus binding to cell membranes. The design of these inhibitors is challenging as many properties, for example, inhibitor size and functionalization with virus attachment factors, strongly influence the inhibition efficiency. Here, virus binding inhibitors are synthesized, the size and functionalization of which are inspired by mucins, which are naturally occurring glycosylated proteins with high molecular weight (MDa range) and interact efficiently with various viruses. Hyperbranched polyglycerols (hPGs) with molecular weights ranging between 10 and 2600 kDa are synthesized, thereby hitting the size of mucins and allowing for determining the impact of inhibitor size on the inhibition efficiency. The hPGs are functionalized with sialic acids and sulfates, as suggested from the structure of mucins, and their inhibition efficiency is determined by probing the inhibition of influenza A virus (IAV) binding to membranes using various methods. The largest, mucin‐sized inhibitor shows potent inhibition at pm concentrations, while the inhibition efficiency decreases with decreasing the molecular weight. Interestingly, the concentration‐dependent IAV inhibition shows a biphasic behavior, which is attributed to differences in the binding affinity of the inhibitors to the two IAV envelope proteins, neuraminidase, and hemagglutinin.
中文翻译:

粘蛋白启发的高分子量病毒结合抑制剂显示与甲型流感病毒的两相结合行为
多价结合抑制剂是一种有前途的新型抗病毒药物,可通过抑制病毒与细胞膜的结合来预防病毒感染。这些抑制剂的设计具有挑战性,因为许多特性(例如抑制剂的大小和病毒附着因子的功能化)会强烈影响抑制效率。在此,合成了病毒结合抑制剂,其大小和功能受到粘蛋白的启发,而粘蛋白是天然存在的具有高分子量(MDa范围)的糖基化蛋白,可与各种病毒有效地相互作用。合成分子量在10到2600 kDa之间的超支化聚甘油(hPG),从而达到粘蛋白的大小,并确定抑制剂大小对抑制效率的影响。如从粘蛋白的结构所暗示的,hPG用唾液酸和硫酸盐官能化,并且它们的抑制效率通过使用各种方法探查对A型流感病毒(IAV)与膜结合的抑制来确定。最大的粘蛋白大小的抑制剂在p处显示出有效的抑制作用米的浓度,同时抑制效率随分子量下降。有趣的是,浓度依赖性IAV抑制表现出双相行为,这归因于抑制剂与两种IAV包膜蛋白,神经氨酸酶和血凝素的结合亲和力的差异。
更新日期:2020-11-27
中文翻译:

粘蛋白启发的高分子量病毒结合抑制剂显示与甲型流感病毒的两相结合行为
多价结合抑制剂是一种有前途的新型抗病毒药物,可通过抑制病毒与细胞膜的结合来预防病毒感染。这些抑制剂的设计具有挑战性,因为许多特性(例如抑制剂的大小和病毒附着因子的功能化)会强烈影响抑制效率。在此,合成了病毒结合抑制剂,其大小和功能受到粘蛋白的启发,而粘蛋白是天然存在的具有高分子量(MDa范围)的糖基化蛋白,可与各种病毒有效地相互作用。合成分子量在10到2600 kDa之间的超支化聚甘油(hPG),从而达到粘蛋白的大小,并确定抑制剂大小对抑制效率的影响。如从粘蛋白的结构所暗示的,hPG用唾液酸和硫酸盐官能化,并且它们的抑制效率通过使用各种方法探查对A型流感病毒(IAV)与膜结合的抑制来确定。最大的粘蛋白大小的抑制剂在p处显示出有效的抑制作用米的浓度,同时抑制效率随分子量下降。有趣的是,浓度依赖性IAV抑制表现出双相行为,这归因于抑制剂与两种IAV包膜蛋白,神经氨酸酶和血凝素的结合亲和力的差异。











































 京公网安备 11010802027423号
京公网安备 11010802027423号