当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unusual substituent effects in the Tr···Te triel bond
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-10-30 , DOI: 10.1002/qua.26526 Ruijing Wang 1 , Canlin Luo 1 , Qingzhong Li 1 , Steve Scheiner 2
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-10-30 , DOI: 10.1002/qua.26526 Ruijing Wang 1 , Canlin Luo 1 , Qingzhong Li 1 , Steve Scheiner 2
Affiliation
|
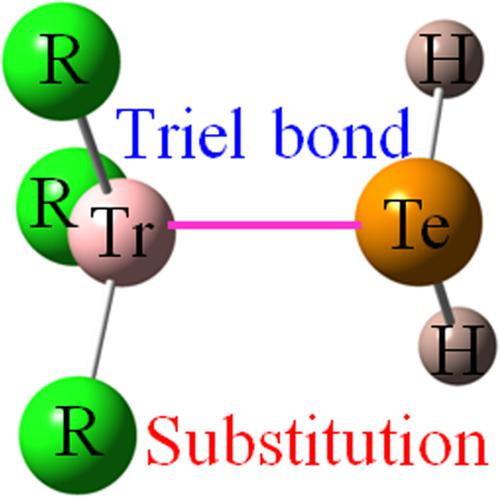
The triel bond between the π‐hole on the triel atom of TrR3 (Tr = B, Al, Ga; R = H, F, Cl, Br) and a lone pair on the Te atom of H2Te is examined using ab initio methods. For Tr = B, the triel bond is weakened as the R substituent becomes more electronegative, while the opposite pattern is noted for Al and Ga. The weakest triel bond of all occurs in H2Te‐BF3 (2.9 kcal/mol) but is much stronger for all the other complexes (>11 kcal/mol). The placement of electron‐releasing OH, NH2, and CH3 R′ substituents on the R′2Te base strengthens the triel bond, whereas the opposite occurs for the electron‐withdrawing CN. Despite its high electronegativity, the F substituent causes a strengthening of the interaction, which is due in large part to the formation of secondary chalcogen bonds that involve the σ‐holes on Te in F2Te. However, the F atom on TrF3 is unable to act as an electron donor in these chalcogen bonds, leading to weakened F2Te··TrF3 interactions.
中文翻译:

Tr···Te triel键中异常的取代基效应
使用ab检验了TrR 3的三元原子(Tr = B,Al,Ga; R = H,F,Cl,Br)上的π孔与H 2 Te的Te原子上的孤对之间的三元键。初始化方法。对于Tr = B,当R取代基变得更具负电性时,三醇键减弱,而对Al和Ga则显示相反的模式。最弱的三醇键出现在H 2 Te-BF 3(2.9 kcal / mol)中,但对于所有其他配合物(> 11 kcal / mol)则要强得多。的电子释放OH,NH放置2,和CH 3 R'的取代基的R' 2Te碱增强了Triel键,而吸电子CN则相反。尽管其高电负性,F取代基仍会增强相互作用,这在很大程度上是由于次级硫族元素键的形成,该元素涉及F 2 Te中Te的σ孔。但是,TrF 3上的F原子不能充当这些硫族元素键中的电子供体,从而导致F 2 Te··TrF 3相互作用减弱。
更新日期:2020-10-30
中文翻译:

Tr···Te triel键中异常的取代基效应
使用ab检验了TrR 3的三元原子(Tr = B,Al,Ga; R = H,F,Cl,Br)上的π孔与H 2 Te的Te原子上的孤对之间的三元键。初始化方法。对于Tr = B,当R取代基变得更具负电性时,三醇键减弱,而对Al和Ga则显示相反的模式。最弱的三醇键出现在H 2 Te-BF 3(2.9 kcal / mol)中,但对于所有其他配合物(> 11 kcal / mol)则要强得多。的电子释放OH,NH放置2,和CH 3 R'的取代基的R' 2Te碱增强了Triel键,而吸电子CN则相反。尽管其高电负性,F取代基仍会增强相互作用,这在很大程度上是由于次级硫族元素键的形成,该元素涉及F 2 Te中Te的σ孔。但是,TrF 3上的F原子不能充当这些硫族元素键中的电子供体,从而导致F 2 Te··TrF 3相互作用减弱。











































 京公网安备 11010802027423号
京公网安备 11010802027423号