Materials Today Bio ( IF 8.7 ) Pub Date : 2020-11-02 , DOI: 10.1016/j.mtbio.2020.100084 Robert Richter 1, 2 , Mohamed A M Kamal 1 , Mariel A García-Rivera 3 , Jerome Kaspar 4 , Maximilian Junk 4 , Walid A M Elgaher 1 , Sanjay Kumar Srikakulam 1 , Alexander Gress 1 , Anja Beckmann 5 , Alexander Grißmer 5 , Carola Meier 5 , Michael Vielhaber 4 , Olga Kalinina 1, 6 , Anna K H Hirsch 1, 2 , Rolf W Hartmann 1 , Mark Brönstrup 3, 7 , Nicole Schneider-Daum 1 , Claus-Michael Lehr 1, 2
|
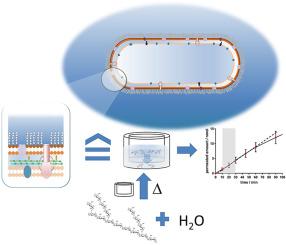
The pipeline of antibiotics has been for decades on an alarmingly low level. Considering the steadily emerging antibiotic resistance, novel tools are needed for early and easy identification of effective anti-infective compounds. In Gram-negative bacteria, the uptake of anti-infectives is especially limited. We here present a surprisingly simple in vitro model of the Gram-negative bacterial envelope, based on 20% (w/v) potato starch gel, printed on polycarbonate 96-well filter membranes. Rapid permeability measurements across this polysaccharide hydrogel allowed to correctly predict either high or low accumulation for all 16 tested anti-infectives in living Escherichia coli. Freeze-fracture TEM supports that the macromolecular network structure of the starch hydrogel may represent a useful surrogate of the Gram-negative bacterial envelope. A random forest analysis of in vitro data revealed molecular mass, minimum projection area, and rigidity as the most critical physicochemical parameters for hydrogel permeability, in agreement with reported structural features needed for uptake into Gram-negative bacteria. Correlating our dataset of 27 antibiotics from different structural classes to reported MIC values of nine clinically relevant pathogens allowed to distinguish active from nonactive compounds based on their low in vitro permeability specifically for Gram-negatives. The model may help to identify poorly permeable antimicrobial candidates before testing them on living bacteria.
中文翻译:

基于水凝胶的体外测定,用于快速预测革兰氏阴性细菌中抗生素的积累
几十年来,抗生素的供应量一直处于惊人的低水平。考虑到不断出现的抗生素耐药性,需要新的工具来早期、轻松地识别有效的抗感染化合物。在革兰氏阴性细菌中,抗感染药物的吸收尤其有限。我们在此提出了一个令人惊讶的简单的革兰氏阴性细菌包膜体外模型,该模型基于 20% (w/v) 马铃薯淀粉凝胶,印刷在聚碳酸酯 96 孔滤膜上。对这种多糖水凝胶的快速渗透性测量可以正确预测所有 16 种测试的抗感染药物在活大肠杆菌中的高或低积累。冷冻断裂 TEM 支持淀粉水凝胶的大分子网络结构可能代表革兰氏阴性细菌包膜的有用替代物。体外数据的随机森林分析显示,分子质量、最小投影面积和刚性是水凝胶渗透性最关键的物理化学参数,与报道的革兰氏阴性细菌吸收所需的结构特征一致。将来自不同结构类别的 27 种抗生素的数据集与九种临床相关病原体报告的 MIC 值相关联,可以根据其低体外渗透性(特别是针对革兰氏阴性菌)区分活性化合物和非活性化合物。该模型可能有助于在对活细菌进行测试之前识别渗透性差的抗菌候选药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号