Journal of Energy Chemistry ( IF 14.0 ) Pub Date : 2020-10-31 , DOI: 10.1016/j.jechem.2020.10.020 Fei Chen , Na Wu , Meixu Zhai , Xue Zhang , Ruihong Guo , Tuoping Hu , Mingming Ma
|
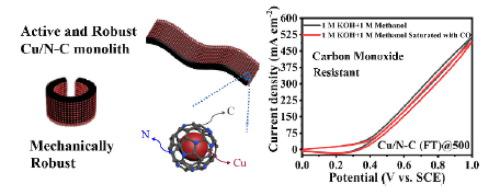
Noble metal-based electrocatalysts present high activities for methanol oxidation reaction (MOR), but are limited by their high cost, low stability and poor resistance to carbon monoxide (CO) poisoning. The development of active and stable non-noble metal electrocatalysts for MOR is desired, but remains as a challenge. Herein, we report a simple strategy to make copper nanocrystal/nitrogen-doped carbon (Cu/N-C) monoliths, which can serve as active and robust electrodes for MOR. Copper nanocrystals were electrochemically deposited onto a conductive polyaniline hydrogel and calcined to form Cu/N-C monolith, where the active copper nanocrystals are protected by nitrogen-doped carbon. Owing to their extremely high electrical conductivity (1.25×105 S cm−1) and mechanical robustness, these Cu/N-C monoliths can be directly used as electrodes for MOR, without using substrates or additives. The optimal Cu/N-C (FT) @500 monolith shows a high MOR activity of 189 mA cm−2 at 0.6 V vs. SCE in alkaline methanol solution, superior to most of reported Cu-based MOR catalysts. Cu/N-C (FT)@500 also presents a better stability than Pt/C catalyst in the long-term MOR test at high current densities. Upon carbon monoxide (CO) poisoning, Cu/N-C (FT)@500 retains 96% of its MOR activity, far exceeding the performance of Pt/C catalyst (61% retention). Owing to its facile synthesis, outstanding activity, high stability and mechanical robustness, Cu/N-C (FT)@500 monolith is promising as a low-cost, efficient and CO-resistant electrocatalyst for MOR.
中文翻译:

坚固的铜纳米晶体/氮掺杂碳单块作为耐一氧化碳的电极用于甲醇氧化反应
贵金属基电催化剂对甲醇氧化反应(MOR)具有很高的活性,但受限于它们的高成本,低稳定性和对一氧化碳(CO)中毒的抵抗力差。期望开发用于MOR的活性和稳定的非贵金属电催化剂,但这仍然是一个挑战。在这里,我们报告了一种简单的策略来制造铜纳米晶体/氮掺杂碳(Cu / NC)整体,可以用作MOR的有源电极。将铜纳米晶体电化学沉积到导电聚苯胺水凝胶上,并煅烧以形成Cu / NC整料,其中活性铜纳米晶体受到氮掺杂碳的保护。由于其极高的电导率(1.25×10 5 S cm -1)和机械坚固性,这些Cu / NC整料可直接用作MOR的电极,而无需使用基材或添加剂。最佳的Cu / NC(FT)@ 500整料在碱性甲醇溶液中在0.6 V电压下相对于SCE具有189 mA cm -2的高MOR活性,优于大多数报道的基于Cu的MOR催化剂。在高电流密度下的长期MOR测试中,Cu / NC(FT)@ 500还表现出比Pt / C催化剂更好的稳定性。一氧化碳(CO)中毒后,Cu / NC(FT)@ 500保留其MOR活性的96%,远远超过Pt / C催化剂的性能(保留61%)。由于其易于合成,出色的活性,高稳定性和机械强度,Cu / NC(FT)@ 500整体料有望成为MOR的低成本,高效且耐CO的电催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号