Colloids and Surfaces B: Biointerfaces ( IF 5.4 ) Pub Date : 2020-10-31 , DOI: 10.1016/j.colsurfb.2020.111436 Małgorzata Nattich-Rak 1 , Agata Pomorska 1 , Piotr Batys 1 , Zbigniew Adamczyk 1
|
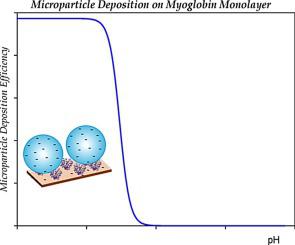
Adsorption kinetics of myoglobin molecules on mica and silica was studied using the atomic force microscopy (AFM), the colloid enhancement and the quartz microbalance (QCM) methods. Measurements were carried out for the NaCl concentration of 0.01 and 0.15 M as a function of pH comprising pH 7.4 stabilized by the PBS buffer. The electrophoretic mobility measurements enabled to derive the molecules zeta potential as a function of pH. The isoelectric point appearing at pH 5, is lower than that predicted from the theoretical calculations of the nominal dissociation charge. The AFM investigations confirmed that myoglobin molecules irreversibly adsorb at pH 3.5 yielding well-defined layers of single molecules. These layers were characterized using the colloid enhancement method involving polymer microparticles for pH range 3–9. The microparticle deposition kinetics was adequately interpreted in terms of a hybrid random sequential adsorption model. It is confirmed that the myoglobin layers exhibit a negligible zeta potential at pH equal to 5 in accordance with the electrophoretic mobility measurements. Analogous adsorption kinetic measurements were performed for the silica substrate using QCM and AFM. It is observed that myoglobin molecules irreversibly adsorb at pH 3.5 forming stable layers of single molecules. On the other hand, its adsorption kinetics at larger pHs was much slower exhibiting a poorly defined maximum coverage. This was attributed to aggregation of the myoglobin solutions due to their vanishing charge. The kinetic QCM runs were adequately interpreted in terms of a theoretical model combining the Smoluchowski aggregation theory with the convective diffusion mass transfer theory.
中文翻译:

肌红蛋白在云母和二氧化硅上的吸附动力学–静电相互作用的作用
使用原子力显微镜(AFM),胶体增强和石英微天平(QCM)方法研究了肌红蛋白分子在云母和二氧化硅上的吸附动力学。进行0.01和0.15M的NaCl浓度作为由PBS缓冲液稳定的pH 7.4的函数的测量,所述pH包括pH 7.4。电泳迁移率测量能够得出分子Zeta电位随pH的变化。pH为5时出现的等电点低于标称解离电荷的理论计算所预测的等电点。原子力显微镜研究证实,肌红蛋白分子在pH 3.5时不可逆地吸附,产生了清晰界定的单分子层。这些层使用胶体增强方法进行表征,该方法涉及pH范围为3–9的聚合物微粒。根据混合随机顺序吸附模型充分解释了微粒沉积动力学。证实了根据电泳迁移率测量,肌红蛋白层在等于5的pH下显示可忽略的ζ电位。使用QCM和AFM对二氧化硅基质进行了类似的吸附动力学测量。观察到肌红蛋白分子在pH 3.5不可逆地吸附,形成稳定的单分子层。另一方面,它在较大pH值下的吸附动力学要慢得多,表现出最大覆盖率的定义不明确。这归因于肌红蛋白溶液由于电荷消失而聚集。











































 京公网安备 11010802027423号
京公网安备 11010802027423号