Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 3.4 ) Pub Date : 2020-10-31 , DOI: 10.1016/j.bbamem.2020.183501 Fahmida Afrose , Ashley N. Martfeld , Denise V. Greathouse , Roger E. Koeppe
|
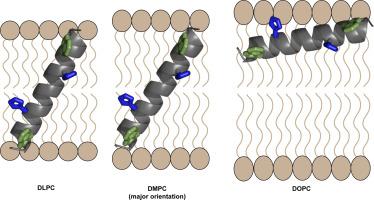
We have employed the peptide framework of GWALP23 (acetyl-GGALWLALALALALALALWLAGA-amide) to examine the orientation, dynamics and pH dependence of peptides having buried single or pairs of histidine residues. When residue L8 is substituted to yield GWALP23-H8, acetyl-GGALWLAH8ALALALALALWLAGA-amide, the deuterium NMR spectra of 2H-labeled core alanine residues reveal a helix that occupies a single transmembrane orientation in DLPC, or in DMPC at low pH, yet shows multiple states at higher pH or in bilayers of DOPC. Moreover, a single histidine at position 8 or 16 in the GWALP23 framework is sensitive to pH. Titration points are observed near pH 3.5 for the deprotonation of H8 in lipid bilayers of DLPC or DMPC, and for H16 in DOPC. When residues L8 and L16 both are substituted to yield GWALP23-H8,16, the 2H NMR spectra show, interestingly, no titration dependence from pH 2–8, yet bilayer thickness-dependent orientation differences. The helix with H8 and H16 is found to adopt a transmembrane orientation in thin bilayers of DLPC, a combination of transmembrane and surface orientations in DMPC, and then a complete transition to a surface bound orientation in the thicker DPoPC and DOPC lipid bilayers. In the surface orientations, alanine A7 no longer fits within the core helix. These results along with previous studies with different locations of histidine residues suggest that lipid hydrophobic thickness is a first determinant and pH a second determinant for the helical orientation, along with possible side-chain snorkeling, when the His residues are incorporated into the hydrophobic region of a lipid membrane-associated helix.
中文翻译:

检查pH依赖性和带有单个或一对掩埋组氨酸残基的跨膜α螺旋的取向差异
我们已经使用了GWALP23(乙酰基-GGALWLALALALALALALALWLAGA-酰胺)的肽框架来检查具有组氨酸残基的单个或成对的肽的方向,动力学和pH依赖性。当取代残基L8生成GWALP23-H 8,乙酰基-GGALWLAH 8 ALALALALALWLAGA-酰胺时,氘核磁共振谱为2H标记的核心丙氨酸残基显示出一个螺旋,该螺旋在DLPC或DMPC中在低pH下占据单个跨膜方向,但在较高pH或在DOPC双层中显示多个状态。此外,在GWALP23框架中第8或16位的单个组氨酸对pH敏感。在DLPC或DMPC的脂质双层中H8的去质子化和在DOPC中的H16的pH值接近3.5时,观察到了滴定点。当残基L8和L16都被取代以产生GWALP23-H 8,16时,2有趣的是,1 H NMR光谱显示不受pH 2-8的滴定依赖性,但取决于双层厚度的方向差异。发现具有H8和H16的螺旋在DLPC的薄双层中采用跨膜取向,在DMPC中采用跨膜和表面取向的组合,然后在较厚的DPoPC和DOPC脂质双层中完全过渡到表面结合的取向。在表面方向上,丙氨酸A7不再适合核心螺旋。这些结果以及对组氨酸残基不同位置的先前研究表明,当将His残基掺入到多肽的疏水区中时,脂质疏水厚度是螺旋取向的第一决定因素,pH是螺旋取向的第二决定因素,可能还有侧链浮潜。脂质膜相关的螺旋。



























 京公网安备 11010802027423号
京公网安备 11010802027423号