Biochimica et Biophysica Acta (BBA) - Bioenergetics ( IF 3.4 ) Pub Date : 2020-10-31 , DOI: 10.1016/j.bbabio.2020.148333 Anna Wójcik-Augustyn , A. Johannes Johansson , Tomasz Borowski
|
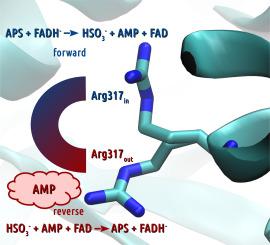
The present research is a continuation of our work on dissimilatory reduction pathway of sulfate - involved in biogeochemical sulfur turnover. Adenosine 5′-phosphosulfate reductase (APSR) is the second enzyme in the dissimilatory pathway of the sulfate to sulfide reduction. It reversibly catalyzes formation of the sulfite anion (HSO3−) from adenosine 5′-phosphosulfate (APS) - the activated form of sulfate provided by ATP sulfurylase (ATPS). Two electrons required for this redox reaction derive from reduced FAD cofactor, which is suggested to be involved directly in the catalysis by formation of FADH-SO3− intermediate. The present work covers quantum-mechanical (QM) studies on APSR reaction performed for eight models of APSR active site. The cluster models were constructed based on two crystal structures (PDB codes: 2FJA and 2FJB), differing in conformation of Arg317 active site residue. The described results indicated the most feasible mechanism of APSR forward reaction, including formation of FADHN-SO3− adduct (with proton on N5 atom of isoalloxazine), tautomerization of FADHN-SO3− to FADHO-SO3− (with proton on C O moiety of isoalloxazine), and its reductive cleavage to oxidized FAD and sulfite anion. The reverse reaction proceeds in the backward direction. It is suggested that it requires two AMP molecules, one acting as a substrate and another as an inhibitor of forward reaction, which forces change of Arg317 conformation from “arginine in” (2FJA) to “arginine out” (2FJB). Important role of Arg317 in switching the course of the APSR catalytic reaction is revealed by changing the direction of thermodynamic driving force. The presented research also shows the importance of the protonation pattern of the reduced FAD cofactor and protein residues within the active site.
O moiety of isoalloxazine), and its reductive cleavage to oxidized FAD and sulfite anion. The reverse reaction proceeds in the backward direction. It is suggested that it requires two AMP molecules, one acting as a substrate and another as an inhibitor of forward reaction, which forces change of Arg317 conformation from “arginine in” (2FJA) to “arginine out” (2FJB). Important role of Arg317 in switching the course of the APSR catalytic reaction is revealed by changing the direction of thermodynamic driving force. The presented research also shows the importance of the protonation pattern of the reduced FAD cofactor and protein residues within the active site.
中文翻译:

异化腺苷5'-磷酸磷酸还原酶催化的反应机理。腺苷5'-单磷酸酯抑制剂和精氨酸317在转换催化过程中的关键作用
本研究是我们对硫酸盐异化还原途径的研究的延续,该途径涉及生物地球化学硫的转化。腺苷5'-磷酸磷酸还原酶(APSR)是硫酸盐还原硫化物异化途径中的第二种酶。它可逆地催化形成亚硫酸盐阴离子(HSO 3 - )选自腺苷-5'-磷酰硫酸(APS) -硫酸盐的活化形式通过提供ATP硫酸(ATPS)。从降低的辅因子FAD此氧化还原反应派生,其被建议可以通过形成的FADH-SO在催化直接涉及需要两个电子3 -中间。本工作涵盖对APSR活性位点的八个模型进行的APSR反应的量子力学(QM)研究。基于两个晶体结构(PDB代码:2FJA和2FJB)构建了簇模型,两个晶体结构的Arg317活性位点残基的构型不同。所描述的结果表明APSR正向反应的最可行的机制,包括形成FADH的Ñ -SO 3 -加合物(用质子上的异咯嗪N5原子),FADH的互变异构Ñ -SO 3 -至FADH ö -SO 3 - (带C质子 异四恶嗪的O部分),并将其还原裂解为氧化的FAD和亚硫酸根阴离子。逆反应向后进行。建议它需要两个AMP分子,一个作为底物,另一个作为正向反应的抑制剂,这迫使Arg317构象从“精氨酸输入”(2FJA)变为“精氨酸输出”(2FJB)。改变热力学驱动力的方向揭示了Arg317在改变APSR催化反应过程中的重要作用。提出的研究还显示了活性位点中还原的FAD辅因子和蛋白质残基的质子化模式的重要性。
异四恶嗪的O部分),并将其还原裂解为氧化的FAD和亚硫酸根阴离子。逆反应向后进行。建议它需要两个AMP分子,一个作为底物,另一个作为正向反应的抑制剂,这迫使Arg317构象从“精氨酸输入”(2FJA)变为“精氨酸输出”(2FJB)。改变热力学驱动力的方向揭示了Arg317在改变APSR催化反应过程中的重要作用。提出的研究还显示了活性位点中还原的FAD辅因子和蛋白质残基的质子化模式的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号