Acta Pharmaceutica Sinica B ( IF 14.7 ) Pub Date : 2020-11-02 , DOI: 10.1016/j.apsb.2020.10.020 Hassan Dana 1 , Ghanbar Mahmoodi Chalbatani 2, 3 , Seyed Amir Jalali 3 , Hamid Reza Mirzaei 2 , Stephan A Grupp 4 , Eloah Rabello Suarez 5 , Catarina Rapôso 6 , Thomas J Webster 7
|
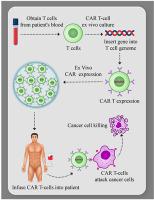
New approaches to cancer immunotherapy have been developed, showing the ability to harness the immune system to treat and eliminate cancer. For many solid tumors, therapy with checkpoint inhibitors has shown promise. For hematologic malignancies, adoptive and engineered cell therapies are being widely developed, using cells such as T lymphocytes, as well as natural killer (NK) cells, dendritic cells, and potentially others. Among these adoptive cell therapies, the most active and advanced therapy involves chimeric antigen receptor (CAR)-T cells, which are T cells in which a chimeric antigen receptor is used to redirect specificity and allow T cell recognition, activation and killing of cancers, such as leukemia and lymphoma. Two autologous CAR-T products have been approved by several health authorities, starting with the U.S. Food and Drug Administration (FDA) in 2017. These products have shown powerful, inducing, long-lasting effects against B cell cancers in many cases. In distinction to the results seen in hematologic malignancies, the field of using CAR-T products against solid tumors is in its infancy. Targeting solid tumors and trafficking CAR-T cells into an immunosuppressive microenvironment are both significant challenges. The goal of this review is to summarize some of the most recent aspects of CAR-T cell design and manufacturing that have led to successes in hematological malignancies, allowing the reader to appreciate the barriers that must be overcome to extend CAR-T therapies to solid tumors successfully.
中文翻译:

CAR-T 细胞:血癌的早期成功和实体瘤的挑战
癌症免疫治疗的新方法已经开发出来,显示出利用免疫系统治疗和消除癌症的能力。对于许多实体瘤,检查点抑制剂治疗已显示出希望。对于血液恶性肿瘤,过继性和工程化细胞疗法正在广泛开发,使用 T 淋巴细胞、自然杀伤 (NK) 细胞、树突状细胞等细胞。在这些过继性细胞疗法中,最活跃和最先进的疗法涉及嵌合抗原受体(CAR)-T细胞,即嵌合抗原受体用于重定向特异性并允许T细胞识别、激活和杀死癌症的T细胞,例如白血病和淋巴瘤。从 2017 年美国食品和药物管理局 (FDA) 开始,两种自体 CAR-T 产品已获得多个卫生当局的批准。这些产品在许多情况下显示出针对 B 细胞癌的强大、诱导、持久的作用。与血液恶性肿瘤中所见的结果不同,使用 CAR-T 产品对抗实体瘤的领域还处于起步阶段。靶向实体瘤和将 CAR-T 细胞运输到免疫抑制微环境都是重大挑战。这篇综述的目的是总结 CAR-T 细胞设计和制造的一些最新方面,这些方面在血液恶性肿瘤方面取得了成功,让读者了解将 CAR-T 疗法扩展到实体瘤中必须克服的障碍。肿瘤成功。










































 京公网安备 11010802027423号
京公网安备 11010802027423号