Acta Pharmaceutica Sinica B ( IF 14.7 ) Pub Date : 2020-10-31 , DOI: 10.1016/j.apsb.2020.10.021 Yihui Song 1 , Min Zhao 1 , Yahong Wu 2 , Bin Yu 1 , Hong-Min Liu 1
|
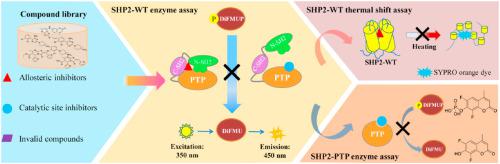
The protein tyrosine phosphatase Src homology phosphotyrosyl phosphatase 2 (SHP2) is implicated in various cancers, and targeting SHP2 has become a promising therapeutic approach. We herein described a robust cross-validation high-throughput screening protocol that combined the fluorescence-based enzyme assay and the conformation-dependent thermal shift assay for the discovery of SHP2 inhibitors. The established method can effectively exclude the false positive SHP2 inhibitors with fluorescence interference and was also successfully employed to identify new protein tyrosine phosphatase domain of SHP2 (SHP2-PTP) and allosteric inhibitors. Of note, this protocol showed potential for identifying SHP2 inhibitors against cancer-associated SHP2 mutation SHP2-E76A. After initial screening of our in-house compound library (∼2300 compounds), we identified 4 new SHP2-PTP inhibitors (0.17% hit rate) and 28 novel allosteric SHP2 inhibitors (1.22% hit rate), of which SYK-85 and WS-635 effectively inhibited SHP2-PTP (SYK-85: IC50 = 0.32 μmol/L; WS-635: IC50 = 4.13 μmol/L) and thus represent novel scaffolds for designing new SHP2-PTP inhibitors. TK-147, an allosteric inhibitor, inhibited SHP2 potently (IC50 = 0.25 μmol/L). In structure, TK-147 could be regarded as a bioisostere of the well characterized SHP2 inhibitor SHP-099, highlighting the essential structural elements for allosteric inhibition of SHP2. The principle underlying the cross-validation protocol is potentially feasible to identify allosteric inhibitors or those inactivating mutants of other proteins.
中文翻译:

多功能交叉验证高通量筛选方案有助于发现新的 SHP2 抑制剂
蛋白质酪氨酸磷酸酶 Src 同源性磷酸酪氨酰磷酸酶 2 (SHP2) 与多种癌症有关,靶向 SHP2 已成为一种有前景的治疗方法。我们在此描述了一种强大的交叉验证高通量筛选方案,该方案结合了基于荧光的酶测定和构象依赖性热位移测定来发现 SHP2 抑制剂。该方法可有效排除具有荧光干扰的假阳性SHP2抑制剂,并成功用于鉴定新的SHP2蛋白酪氨酸磷酸酶结构域(SHP2-PTP)和变构抑制剂。值得注意的是,该方案显示了识别针对癌症相关 SHP2 突变 SHP2-E76A 的 SHP2 抑制剂的潜力。经过对我们内部化合物库(约 2300 种化合物)的初步筛选,我们鉴定了 4 种新的 SHP2-PTP 抑制剂(命中率 0.17%)和 28 种新型变构 SHP2 抑制剂(命中率 1.22%),其中 SYK-85 和 WS -635有效抑制SHP2-PTP(SYK-85:IC 50 = 0.32 μmol/L;WS-635:IC 50 = 4.13 μmol/L),因此代表了设计新SHP2-PTP抑制剂的新支架。 TK-147 是一种变构抑制剂,可有效抑制 SHP2 (IC 50 = 0.25 μmol/L)。在结构上,TK-147 可被视为已充分表征的 SHP2 抑制剂 SHP-099 的生物电子等排体,突出了 SHP2 变构抑制的重要结构元件。交叉验证方案的基本原理对于识别变构抑制剂或其他蛋白质的失活突变体可能是可行的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号