Letters in Drug Design & Discovery ( IF 1.2 ) Pub Date : 2020-10-31 , DOI: 10.2174/1570180817999200623115049 Vinutha Vittala Salian 1 , Badiadka Narayana 1 , Balladka Kunhanna Sarojini 2 , Sharath Chandra Kodandoor 3 , Anupam Glorious Lobo 4
|
Background: Development of potential antimicrobial agents is the main aim in the drug discovery process to overcome the problem of drug resistance. Pyrazolines and thiazolinones are extensively used as building blocks for the synthesis of diverse and medicinally important compounds.
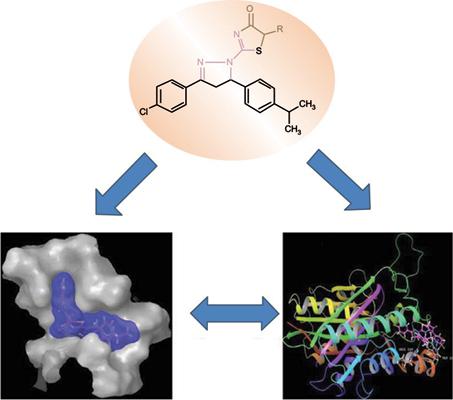
Methods: In this present work, a new series of functionalized pyrazolinyl-thiazolinone biheterocycles is designed and synthesized from N-pyrazolinecarbothioamide. Antimicrobial screening is carried out in order to discover their potential towards six bacterial and four fungal strains. The zone of inhibition (ZI in mm) was determined by the disc diffusion method and minimum inhibitory concentration (MIC in μg/mL) by macro dilution method. The druggability of these new entities is done through in silico pharmacokinetic profiling using Maestro 2017-1 interface of Schrӧdinger software.
Results and Disscusion: Compounds 4c and 4e with chloro and iodo substituents on Nphenylacetamide ring displayed good inhibitory antibacterial activity against the tested bacterial strains with minimum MIC values when compared to the reference drug tetracycline. Compound 3 with an acetic acid derivative showed high antifungal activity among all the tested derivatives. Compound 3 not only showed antifungal activity but also qualified druggability test with no violation of Lipinski rule of five.
Conclusion: The capability of the synthesized pyrazolinyl-thiazolinone derivatives was performed to efficiently inhibit the growth of microorganisms against selected bacterial and fungal strains. Further, these compounds are found to be effectively bound to the active sites of attractive target Escherichia coli FabH.
中文翻译:

新型吡唑啉基噻唑啉酮双杂环作为有效抗菌剂的设计,合成,对接和计算药代动力学分析
背景:潜在的抗菌剂的开发是药物开发过程中克服耐药性问题的主要目标。吡唑啉和噻唑啉酮被广泛用作合成各种重要的药用化合物的基础。
方法:在本工作中,由N-吡唑啉碳硫酰胺设计并合成了一系列新的功能化吡唑啉基-噻唑啉酮双杂环。进行抗菌筛选以发现其对六种细菌和四种真菌菌株的潜力。通过盘扩散法确定抑制区域(ZI以毫米为单位),通过宏观稀释法确定最小抑制浓度(MIC以μg/ mL为单位)。这些新实体的可药用性通过使用Schrӧdinger软件的Maestro 2017-1界面通过计算机进行药物动力学分析来完成。
结果与讨论:与参考药物四环素相比,在N苯基乙酰胺环上具有氯和碘取代基的化合物4c和4e对被测细菌菌株显示出良好的抑菌活性,且MIC值最小。在所有测试的衍生物中,具有乙酸衍生物的化合物3显示出高的抗真菌活性。化合物3不仅显示出抗真菌活性,而且还进行了合格的药物测试,没有违反Lipinski的5条法则。
结论:合成的吡唑啉基-噻唑啉酮衍生物具有有效抑制所选菌株和真菌对微生物生长的能力。此外,发现这些化合物有效地结合至有吸引力的靶大肠杆菌FabH的活性位点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号