Letters in Drug Design & Discovery ( IF 1.2 ) Pub Date : 2020-10-31 , DOI: 10.2174/1570180817999200618162949 Si Young Sung 1 , Yu Na Chae 2 , Dae Young Lee 2 , Kyeong Min Kim 2 , Eun Jung Kim 2 , Ji Hye Han 1 , Wook Kim 1 , Sung-Hwa Yoon 1
|
Background: Dapagliflozin, developed as an SGLT-2 inhibitor, has a low melting point and high hygroscopicity, which needs extreme care during pharmaceutical production to keep the active pharmacological property. Various attempts have been made to overcome these problematic properties.
Objectives: To develop dapagliflozin prodrugs that have similar pharmacological effects with improved hygroscopicity and thermal stability.
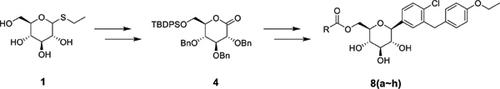
Methods: The novel dapagliflozin ester prodrugs containing pharmaceutically acceptable moieties were synthesized and their pharmacokinetics (PK) and physical properties were compared with dapagliflozin propanediol hydrate (DPD, Farxiga®). The PK in dog and rat, in vitro stability, hygroscopicity, and physical property studies in accelerated conditions (40°C, 75% RH) were performed with prodrugs.
Results and Discussions: Among the eight synthesized prodrugs, Cmax and AUC0-48h values of prodrug 8b (1.35 μg/ml and 14.78 μg·h/ml, respectively) were similar to those of DPD (1.67 μg/ml and 14.27 μg·h/ml, respectively). However, the rest of the prodrugs 8a, 8c, 8d, 8e, 8f, 8g and 8h showed significantly lower Cmax and AUC0-48h values than DPD. Prodrug 8b completely converted into parent drug in the body.
Conclusion: The novel prodrug 8b exhibited comparative PK profile to that of DPD, but with low hygroscopic property and better thermal stability than DPD.
中文翻译:

具有改善的吸湿性和热稳定性的达格列净酯前药的合成与评价
背景:Dapagliflozin作为SGLT-2抑制剂而开发,具有低熔点和高吸湿性,在药物生产过程中需要格外小心以保持活性药理特性。已经进行了各种尝试来克服这些有问题的特性。
目的:开发达格列净前药,这些药具有相似的药理作用,并具有改善的吸湿性和热稳定性。
方法:合成含有药物可接受部分的新型达格列净酯前药,并与达格列净丙二醇水合物(DPD,Farxiga®)比较其药代动力学(PK)和物理性质。用前药在加速条件下(40°C,75%RH)进行了犬和大鼠的PK,体外稳定性,吸湿性和物理性质研究。
结果与讨论:在8种合成前药中,前药8b的Cmax和AUC0-48h值(分别为1.35μg/ ml和14.78μg·h / ml)与DPD相似(1.67μg/ ml和14.27μg·h) / ml)。但是,其余的前药8a,8c,8d,8e,8f,8g和8h的Cmax和AUC0-48h值均明显低于DPD。前药8b在体内完全转化为母体药物。
结论:新型前药8b的PK曲线与DPD相当,但吸湿性低,热稳定性优于DPD。











































 京公网安备 11010802027423号
京公网安备 11010802027423号