Letters in Drug Design & Discovery ( IF 1.2 ) Pub Date : 2020-10-31 , DOI: 10.2174/2352096513999200714103641 Sen-Chuan Song 1 , Yu-Liang Mai 1 , Hua-Hong Shi 1 , Bing Liao 1 , Fei Wang 1
|
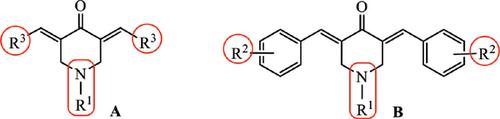
Background: Inhibition of cancer cell growth and low in vivo toxicity are two important criteria for the development of anti-cancer drugs. Curcumin is a promising candidate for developing novel anti-cancer drug analogs. The research group designed the 3,5-bis-(3,4,5- trimethoxybenzylidene)-1-methyl-piperidin-4-one analog of curcumin that significantly inhibited the growth of esophageal cancer cells in vivo. In this study, 81 curcumin analogs were synthesized, analyzed both in vitro and in vivo, and their structure activity relationships (SARs) were determined.
Methods: Based on the parent structure of curcumin, 81 analogs of 3,5-bis(substitutedbenzylidene)- piperidin-4-one compounds were designed and synthesized. Their anti-cancer activity in the human cancer cell lines was evaluated using the MTT assay, and in vivo toxicity was evaluated in mice. The SARs of selected compounds were analyzed.
Results and Discussion: Among the designed curcumin analogs, 61 compounds exerted anti-cancer effects higher than the parent compound in vitro; 23 compounds inhibited cell growth in the human cancer cell line at low concentrations (IC50 values below 1 μM). The acute toxicity of curcumin analogs was tested in mice; 13 compounds were selected, which did not show any obvious toxicity at doses as high as 25.0 mg/kg. The SARs of these shortlisted curcumin analogs were determined.
Conclusion: Twenty-three curcumin analogs exhibiting promising in vitro anti-cancer activity and low in vivo toxicity were designed. SAR analysis indicated the optimal functional groups in the molecule required for anti-cancer activity. This study not only suggested a useful strategy to design curcumin analogs for the development of anti-cancer drugs, but also revealed a group of curcumin analogs which could be further explored.
中文翻译:

姜黄素类似物的高抗癌活性,低动物毒性和结构活性关系
背景:抑制癌细胞生长和降低体内毒性是开发抗癌药物的两个重要标准。姜黄素是开发新型抗癌药物类似物的有希望的候选者。该研究小组设计了姜黄素的3,5-双-(3,4,5-三甲氧基亚苄基)-1-甲基-哌啶-4-一类似物,该类似物可显着抑制体内食管癌细胞的生长。在这项研究中,合成了81个姜黄素类似物,在体内外进行了分析,并确定了它们的结构活性关系(SAR)。
方法:根据姜黄素的母体结构,设计合成了3,5-双(取代亚苄基)-哌啶-4-酮化合物的81个类似物。使用MTT分析评估了它们在人类癌细胞系中的抗癌活性,并评估了小鼠体内毒性。分析了所选化合物的SAR。
结果与讨论:在设计的姜黄素类似物中,有61种化合物在体外具有比母体化合物更高的抗癌作用。23种化合物以低浓度(低于1μM的IC50值)抑制人癌细胞系中的细胞生长。在小鼠中测试了姜黄素类似物的急性毒性。选择了13种化合物,它们在高达25.0 mg / kg的剂量下均未显示任何明显的毒性。确定了这些入围姜黄素类似物的SAR。
结论:设计了二十三种姜黄素类似物,它们具有良好的体外抗癌活性和低体内毒性。SAR分析表明,抗癌活性需要分子中的最佳官能团。这项研究不仅提出了设计姜黄素类似物以开发抗癌药物的有用策略,而且揭示了一组姜黄素类似物有待进一步研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号