Current Analytical Chemistry ( IF 1.8 ) Pub Date : 2020-11-30 , DOI: 10.2174/1573411016999200802024151 Mohammad AlZeer 1 , Ibrahim A. Darwish 1 , Nasr Y. Khalil 1
|
Background: Axitinib (AXT) is a member of the new generation of the kinase inhibitor indicated for the treatment of advanced renal cell carcinoma. Its therapeutic benefits depend on assuring the good-quality of its dosage forms in terms of content and stability of the pharmaceutically active ingredient.
Objective: This study was devoted to the development of a simple, sensitive and accurate stabilityindicating high-performance liquid chromatographic method with ultraviolet detection (HPLC-UV) for the determination of AXT in its bulk and dosage forms.
Methods: Waters HPLC system was used. The chromatographic separation of AXT, internal standard (olaparib), and degradation products were performed on the Nucleosil CN column (250 × 4.6 mm, 5 μm). The mobile phase consisted of water:acetonitrile:methanol (40:40:20, v/v/v) with a flow rate of 1 ml/min, and the UV detector was set at 225 nm. AXT was subjected to different accelerated stress conditions and the degradation products, when any, were completely resolved from the intact AXT.
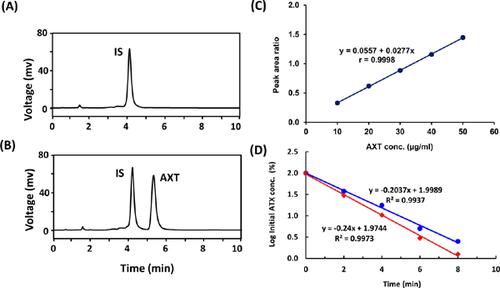
Results: The method was linear (r = 0.9998) in the concentration range of 5-50 μg/ml. The limits of detection and quantitation were 0.85 and 2.57 μg/ml, respectively. The accuracy of the method, measured as recovery, was in the range of 98.0-103.6% with relative standard deviations in the range of 0.06-3.43%. The results of stability testing revealed that AXT was mostly stable in neutral and oxidative conditions; however, it was unstable in alkaline and acidic conditions. The kinetics of degradation were studied, and the kinetic rate constants were determined. The proposed method was successfully applied for the determination of AXT in bulk drug and dosage forms.
Conclusion: A stability-indicating HPLC-UV method was developed and validated for assessing AXT stability in its bulk and dosage forms. The method met the regulatory requirements of the International Conference on Harmonization (ICH) and the Food and Drug Administration (FDA). The results demonstrated that the method would have great value when applied in quality control and stability studies for AXT.
中文翻译:

符合ICH / FDA准则的经验证的稳定性指示HPLC-UV方法,用于测定散装和剂型阿昔替尼
背景:阿昔替尼(AXT)是新一代激酶抑制剂的成员,该激酶抑制剂可用于治疗晚期肾细胞癌。就药物活性成分的含量和稳定性而言,其治疗益处取决于确保其剂型的优良品质。
目的:本研究致力于开发一种简单,灵敏,准确的稳定性指示高效液相色谱法,该方法采用紫外检测(HPLC-UV)测定散装和剂型中的AXT。
方法:使用Waters HPLC系统。在Nucleosil CN色谱柱(250×4.6 mm,5μm)上进行AXT,内标(olaparib)和降解产物的色谱分离。流动相由水:乙腈:甲醇(40:40:20,v / v / v)组成,流速为1 ml / min,UV检测器设置为225 nm。AXT经受了不同的加速应力条件,并且降解产物(如果有的话)可以从完整的AXT中完全分解出来。
结果:该方法在5-50μg/ ml的浓度范围内是线性的(r = 0.9998)。检测限和定量限分别为0.85和2.57μg/ ml。以回收率衡量,该方法的准确性在98.0-103.6%的范围内,相对标准偏差在0.06-3.43%的范围内。稳定性测试结果表明,AXT在中性和氧化条件下大多数情况下是稳定的。但是,在碱性和酸性条件下不稳定。研究了降解动力学,并确定了动力学速率常数。所提出的方法已成功应用于散装药物和剂型中AXT的测定。
结论:建立了指示稳定性的HPLC-UV方法,并验证了其在AXT的体积和剂型中的稳定性。该方法符合国际协调会议(ICH)和食品药品管理局(FDA)的法规要求。结果表明,该方法在AXT质量控制和稳定性研究中具有重要的应用价值。



























 京公网安备 11010802027423号
京公网安备 11010802027423号