当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Decomposition of Vanadium(V) Alkylidenes Relevant to Olefin Metathesis
Organometallics ( IF 2.5 ) Pub Date : 2020-10-30 , DOI: 10.1021/acs.organomet.0c00610 Wesley S. Farrell 1 , Christine Greene 2 , Pokhraj Ghosh 2 , Timothy H. Warren 2 , Peter Y. Zavalij 3
Organometallics ( IF 2.5 ) Pub Date : 2020-10-30 , DOI: 10.1021/acs.organomet.0c00610 Wesley S. Farrell 1 , Christine Greene 2 , Pokhraj Ghosh 2 , Timothy H. Warren 2 , Peter Y. Zavalij 3
Affiliation
|
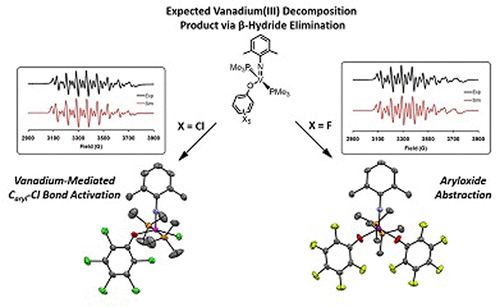
Vanadium alkylidenes can be highly active initiators for ring-opening metathesis polymerization of cyclic olefins; however, attempts to expand their use to cross metathesis have been unsuccessful due to catalyst decomposition. Detailed knowledge of the decomposition reaction is imperative to guide future catalyst design. Herein, we demonstrate that β-hydride elimination is the dominant decomposition pathway during cross metathesis. The isolated vanadium decomposition products [N-2,6-(CH3)2C6H3](OC6Cl5)[P(CH3)3]2VCl (10) and [N-2,6-(CH3)2C6H3]V(OC6F5)2[P(CH3)3]2 (11), generated from two separate catalysts, agree with this pathway. Compounds 10 and 11 were shown computationally to form via bimolecular routes after β-hydride elimination from the metallocyclobutane and reductive elimination of propylene, which was itself demonstrated to be exergonic with low thermodynamic barriers to metallocyclobutane formation. Relative conversions in the self-metathesis of 1-hexene with the series of vanadium alkylidenes of various sterics and electronics (N-2,6-X2C6H3)(OC6Y5)[P(CH3)3]2V[CHSi(CH3)3] [X = CH3, Y = Cl (1); X = CH(CH3)2, Y = Cl (6), X = CH(CH3)2, Y = F (7)] were determined. It was found that bulkier, yet more electron-donating ligands decreased conversions, indicating that β-hydride elimination is favored over bimolecular decomposition. Analysis of organic reaction products demonstrated that reductive elimination of propylene occurs before insertion of ethylene into the newly formed vanadium–hydrogen bond.
中文翻译:

与烯烃复分解有关的钒(V)炔烃的分解
钒亚烷基可以是高活性的引发剂,用于环烯烃的开环易位聚合。然而,由于催化剂分解,尝试扩大其用途以进行交叉复分解的努力并未成功。必须详细了解分解反应,以指导将来的催化剂设计。在本文中,我们证明了β-氢化物消除是交叉复分解期间的主要分解途径。分离出的钒分解产物[N-2,6-(CH 3)2 C 6 H 3 ](OC 6 Cl 5)[P(CH 3)3 ] 2 VCl(10)和[N-2,6-( CH 3)2由两种单独的催化剂生成的C 6 H 3 ] V(OC 6 F 5)2 [P(CH 3)3 ] 2(11)与该途径一致。在金属环丁烷中将β-氢化物消除并通过丙烯的还原性消除后,通过计算显示化合物10和11是通过双分子途径形成的,丙烯本身被证明具有能动性,对金属环丁烷的形成具有较低的热力学势垒。1-己烯自我复分解与各种空间和电子(N-2,6-X 2 C 6)系列钒亚烷基的相对转化H 3)(OC 6 Y 5)[P(CH 3)3 ] 2 V [CHSi(CH 3)3 ] [X = CH 3,Y = Cl(1); X = CH(CH 3)2,Y = Cl(6),X = CH(CH 3)2,Y = F(7)]确定。发现更大的,但更多的供电子配体降低了转化率,表明β-氢化物的消除优于双分子分解。对有机反应产物的分析表明,在将乙烯插入新形成的钒氢键之前,要进行丙烯的还原消除。
更新日期:2020-11-09
中文翻译:

与烯烃复分解有关的钒(V)炔烃的分解
钒亚烷基可以是高活性的引发剂,用于环烯烃的开环易位聚合。然而,由于催化剂分解,尝试扩大其用途以进行交叉复分解的努力并未成功。必须详细了解分解反应,以指导将来的催化剂设计。在本文中,我们证明了β-氢化物消除是交叉复分解期间的主要分解途径。分离出的钒分解产物[N-2,6-(CH 3)2 C 6 H 3 ](OC 6 Cl 5)[P(CH 3)3 ] 2 VCl(10)和[N-2,6-( CH 3)2由两种单独的催化剂生成的C 6 H 3 ] V(OC 6 F 5)2 [P(CH 3)3 ] 2(11)与该途径一致。在金属环丁烷中将β-氢化物消除并通过丙烯的还原性消除后,通过计算显示化合物10和11是通过双分子途径形成的,丙烯本身被证明具有能动性,对金属环丁烷的形成具有较低的热力学势垒。1-己烯自我复分解与各种空间和电子(N-2,6-X 2 C 6)系列钒亚烷基的相对转化H 3)(OC 6 Y 5)[P(CH 3)3 ] 2 V [CHSi(CH 3)3 ] [X = CH 3,Y = Cl(1); X = CH(CH 3)2,Y = Cl(6),X = CH(CH 3)2,Y = F(7)]确定。发现更大的,但更多的供电子配体降低了转化率,表明β-氢化物的消除优于双分子分解。对有机反应产物的分析表明,在将乙烯插入新形成的钒氢键之前,要进行丙烯的还原消除。











































 京公网安备 11010802027423号
京公网安备 11010802027423号