当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Augmented Hückel molecular orbital model of π‐electron systems: from topology to metric. I. General theory
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-10-27 , DOI: 10.1002/poc.4154 Leszek Z. Stolarczyk 1 , T. Marek Krygowski 1
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-10-27 , DOI: 10.1002/poc.4154 Leszek Z. Stolarczyk 1 , T. Marek Krygowski 1
Affiliation
|
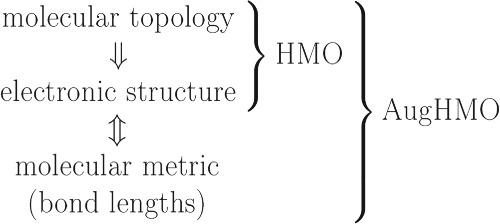
The π‐electron molecule is planar (but the strict planarity requirement may be relaxed), with a set of electrons residing on the (valence) molecular orbitals of the π‐symmetry (πMOs). The quantum state of these electrons influences the geometry of the molecule, most notably the bond lengths. The Hückel molecular orbital (HMO) model provides the simplest (yet quite effective) description of the πMOs. Longuet‐Higgins and Salem, and their followers, made efforts to augment this model by making its empirical parameters dependent on the bond lengths; this approach was later often referred to as the SCβ HMO model. In the present paper, we introduce the AugHMO model that is a generalization of these earlier efforts. We show that the mathematical formulation of this model necessarily implies the principle of a bond‐order–bond‐length (BO‐BL) relationship that is universal for a given type of bond (C–C, C–N, etc.) in all π‐electron molecules. A plausible assumption that the BO‐BL relationship is a linear one leads to a family of workable computational methods, such as the Hückel–Su–Schrieffer–Heeger method and the Hückel–Longuet–Higgins–Salem method. Our AugHMO model is developed with the following goals in mind: (i) to offer a predictive tool for calculating the bond lengths in π‐electron molecules, capable of dealing with very large systems; (ii) to provide insights into the problem of coupling between the π‐electrons and the bond lengths; and (iii) to offer a simple educational model illustrating the principles of geometry optimization in quantum chemistry (involving the gradient and Hessian of the molecular energy).
中文翻译:

π电子系统的增强Hückel分子轨道模型:从拓扑到度量。一,通论
所述π π电子分子是平面的(但严格的平坦性要求可放宽),具有一组驻留在(化合价)的电子的分子轨道的π -symmetry(π MOS)。这些电子的量子态会影响分子的几何形状,尤其是键长。休克尔分子轨道(HMO)模型提供了最简单的(但非常有效的)描述π的MO。Longuet-Higgins和Salem及其追随者通过使模型的经验参数取决于键的长度来努力扩展该模型。这种方法后来通常称为SCβHMO模型。在本文中,我们介绍了AugHMO模型,该模型是这些早期工作的概括。我们表明,该模型的数学公式必然暗示了键序-键长(BO-BL)关系的原理,该关系对于给定类型的键(CC,CN等)是通用的所有 π电子分子。BO-BL关系是线性关系的合理假设导致了一系列可行的计算方法,例如Hückel–Su–Schrieffer–Heeger方法和Hückel–Longuet–Higgins-Salem方法。我们的AugHMO模型的开发考虑了以下目标:(i)提供一种预测工具来计算π中的键长电子分子,能够处理非常大的系统;(ii)提供有关π电子与键长之间耦合问题的见解;(iii)提供一个简单的教学模型,说明量子化学中几何优化的原理(涉及分子能量的梯度和Hessian)。
更新日期:2020-10-27
中文翻译:

π电子系统的增强Hückel分子轨道模型:从拓扑到度量。一,通论
所述π π电子分子是平面的(但严格的平坦性要求可放宽),具有一组驻留在(化合价)的电子的分子轨道的π -symmetry(π MOS)。这些电子的量子态会影响分子的几何形状,尤其是键长。休克尔分子轨道(HMO)模型提供了最简单的(但非常有效的)描述π的MO。Longuet-Higgins和Salem及其追随者通过使模型的经验参数取决于键的长度来努力扩展该模型。这种方法后来通常称为SCβHMO模型。在本文中,我们介绍了AugHMO模型,该模型是这些早期工作的概括。我们表明,该模型的数学公式必然暗示了键序-键长(BO-BL)关系的原理,该关系对于给定类型的键(CC,CN等)是通用的所有 π电子分子。BO-BL关系是线性关系的合理假设导致了一系列可行的计算方法,例如Hückel–Su–Schrieffer–Heeger方法和Hückel–Longuet–Higgins-Salem方法。我们的AugHMO模型的开发考虑了以下目标:(i)提供一种预测工具来计算π中的键长电子分子,能够处理非常大的系统;(ii)提供有关π电子与键长之间耦合问题的见解;(iii)提供一个简单的教学模型,说明量子化学中几何优化的原理(涉及分子能量的梯度和Hessian)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号