当前位置:
X-MOL 学术
›
Chem. Rec.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Asymmetric Total Synthesis of Leucinostatin A
The Chemical Record ( IF 7.0 ) Pub Date : 2020-10-27 , DOI: 10.1002/tcr.202000108 Takumi Watanabe 1 , Hikaru Abe 1 , Masakatsu Shibasaki 1
The Chemical Record ( IF 7.0 ) Pub Date : 2020-10-27 , DOI: 10.1002/tcr.202000108 Takumi Watanabe 1 , Hikaru Abe 1 , Masakatsu Shibasaki 1
Affiliation
|
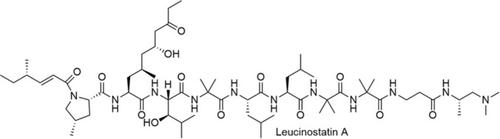
This review describes our efforts toward achieving catalytic asymmetric total synthesis of leucinostatin A, a compound that interferes with the tumor‐stroma interaction. The synthesis utilizes four catalytic asymmetric reactions, including direct‐type reactions exemplified by high atom‐economy, and three C−C bond forming reactions. Thorough analysis of the NMR data, HPLC profiles, and biologic activity led us to unambiguously revise the absolute configuration regarding the 6‐position of the AHMOD residue side chain from S (reported) to R. Other examples of previously reported important studies on the stereoselective synthesis of HyLeu and AHMOD are also described.
中文翻译:

芥子抑素A的催化不对称全合成
这篇综述描述了我们为实现亮氨酸抑制素A的催化不对称全合成而做出的努力,亮氨酸抑素A是一种干扰肿瘤基质相互作用的化合物。该合成利用了四个催化不对称反应,包括以高原子经济为例的直接型反应和三个C键形成反应。对NMR数据,HPLC谱图和生物活性进行了透彻的分析,使我们明确修改了AHMOD残基侧链6位从S(报告)到R的绝对构型。还描述了先前报道的有关HyLeu和AHMOD的立体选择性合成的重要研究的其他实例。
更新日期:2020-10-27
中文翻译:

芥子抑素A的催化不对称全合成
这篇综述描述了我们为实现亮氨酸抑制素A的催化不对称全合成而做出的努力,亮氨酸抑素A是一种干扰肿瘤基质相互作用的化合物。该合成利用了四个催化不对称反应,包括以高原子经济为例的直接型反应和三个C键形成反应。对NMR数据,HPLC谱图和生物活性进行了透彻的分析,使我们明确修改了AHMOD残基侧链6位从S(报告)到R的绝对构型。还描述了先前报道的有关HyLeu和AHMOD的立体选择性合成的重要研究的其他实例。











































 京公网安备 11010802027423号
京公网安备 11010802027423号