Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stimulation of GMP formation in hGBP1 is mediated by W79 and its effect on the antiviral activity
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-10-28 , DOI: 10.1111/febs.15611 Nikunj Raninga 1 , Shahid M Nayeem 2 , Sowmiya Gupta 1 , Ranajoy Mullick 3 , Esha Pandita 1 , Saumitra Das 3 , Shashank Deep 4 , Apurba Kumar Sau 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-10-28 , DOI: 10.1111/febs.15611 Nikunj Raninga 1 , Shahid M Nayeem 2 , Sowmiya Gupta 1 , Ranajoy Mullick 3 , Esha Pandita 1 , Saumitra Das 3 , Shashank Deep 4 , Apurba Kumar Sau 1
Affiliation
|
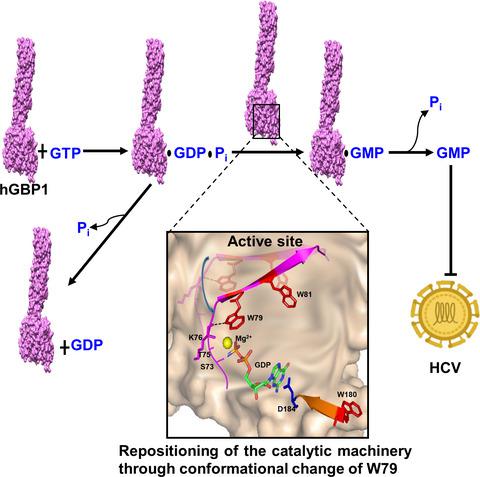
Interferon‐inducible large GTPases are critical for innate immunity. The distinctive feature of a large GTPase, human guanylate binding protein‐1 (hGBP1), is the sequential hydrolysis of GTP into GMP via GDP. Despite several structural and biochemical studies, the underlying mechanism of assembly‐stimulated GMP formation by hGBP1 and its role in immunity are not fully clarified. Using a series of biochemical, biophysical, and in silico experiments, we studied four tryptophan residues, located near switch I‐II (in and around the active site) to understand the conformational changes near these regions and also to investigate their effect on enhanced GMP formation. The W79A mutation showed significantly reduced GMP formation, whereas the W81A and W180A substitutions exhibited only a marginal defect. The W114A mutation showed a long‐range effect of further enhanced GMP formation, which was mediated through W79. We also observed that after first phosphate cleavage, the W79‐containing region undergoes a conformational change, which is essential for stimulated GMP formation. We suggest that this conformational change helps to reposition the active site for the next cleavage step, which occurs through a stable contact between the indole moiety of W79 and the main chain carbonyl of K76. We also showed that stimulated GMP formation is crucial for antiviral activity against hepatitis C. Thus, the present study not only provides new insight for the stimulation of GMP formation in hGBP1, but also highlights the importance of the enhanced second phosphate cleavage product in the antiviral activity.
中文翻译:

WGB介导hGBP1中GMP的形成及其对抗病毒活性的影响
干扰素诱导的大GTP酶对于先天免疫至关重要。大型鸟苷酸结合蛋白-1(hGBP1)是大型GTP酶的显着特征,即GTP可以通过GDP顺序水解为GMP。尽管进行了一些结构和生化研究,但hGBP1组装刺激GMP形成的基本机制及其在免疫中的作用尚未完全阐明。使用一系列生化,生物物理和计算机模拟实验中,我们研究了位于开关I‐II附近(在活性位点内和周围)的四个色氨酸残基,以了解这些区域附近的构象变化,并研究它们对增强GMP形成的影响。W79A突变显示GMP形成明显减少,而W81A和W180A替代仅表现出边缘缺陷。W114A突变显示出进一步增强的GMP形成的长期效应,这是通过W79介导的。我们还观察到,第一次磷酸酯切割后,含有W79的区域发生构象变化,这对刺激GMP的形成至关重要。我们建议这种构象变化有助于重新定位下一个切割步骤的活性位点,该步骤通过W79的吲哚部分与K76的主链羰基之间的稳定接触而发生。
更新日期:2020-10-28
中文翻译:

WGB介导hGBP1中GMP的形成及其对抗病毒活性的影响
干扰素诱导的大GTP酶对于先天免疫至关重要。大型鸟苷酸结合蛋白-1(hGBP1)是大型GTP酶的显着特征,即GTP可以通过GDP顺序水解为GMP。尽管进行了一些结构和生化研究,但hGBP1组装刺激GMP形成的基本机制及其在免疫中的作用尚未完全阐明。使用一系列生化,生物物理和计算机模拟实验中,我们研究了位于开关I‐II附近(在活性位点内和周围)的四个色氨酸残基,以了解这些区域附近的构象变化,并研究它们对增强GMP形成的影响。W79A突变显示GMP形成明显减少,而W81A和W180A替代仅表现出边缘缺陷。W114A突变显示出进一步增强的GMP形成的长期效应,这是通过W79介导的。我们还观察到,第一次磷酸酯切割后,含有W79的区域发生构象变化,这对刺激GMP的形成至关重要。我们建议这种构象变化有助于重新定位下一个切割步骤的活性位点,该步骤通过W79的吲哚部分与K76的主链羰基之间的稳定接触而发生。











































 京公网安备 11010802027423号
京公网安备 11010802027423号